Aconitase
| aconitate hydratase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Illustration of pig aconitase in complex with the [Fe4S4] cluster. The protein is colored by secondary structure, and iron atoms are blue and the sulfur red.[1] | |||||||||
| Identifiers | |||||||||
| EC number | 4.2.1.3 | ||||||||
| CAS number | 9024-25-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
| Aconitase family (aconitate hydratase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Structure of aconitase.[2] | |||||||||
| Identifiers | |||||||||
| Symbol | Aconitase | ||||||||
| Pfam | PF00330 | ||||||||
| InterPro | IPR001030 | ||||||||
| PROSITE | PDOC00423 | ||||||||
| SCOP | 1aco | ||||||||
| SUPERFAMILY | 1aco | ||||||||
| |||||||||
Aconitase (aconitate hydratase; EC 4.2.1.3) is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle, a non-redox-active process.[3][4][5]
Structure
Aconitase, displayed in the structures in the right margin of this page, has two slightly different structures, depending on whether it is activated or inactivated.[6][7] In the inactive form, its structure is divided into four domains.[6] Counting from the N-terminus, only the first three of these domains are involved in close interactions with the [3Fe-4S] cluster, but the active site consists of residues from all four domains, including the larger C-terminal domain.[6] The Fe-S cluster and a SO42− anion also reside in the active site.[6] When the enzyme is activated, it gains an additional iron atom, creating a [4Fe-4S] cluster.[7][8] However, the structure of the rest of the enzyme is nearly unchanged; the conserved atoms between the two forms are in essentially the same positions, up to a difference of 0.1 angstroms.[7]
Function
In contrast with the majority of iron-sulfur proteins that function as electron carriers, the iron-sulfur cluster of aconitase reacts directly with an enzyme substrate. Aconitase has an active [Fe4S4]2+ cluster, which may convert to an inactive [Fe3S4]+ form. Three cysteine (Cys) residues have been shown to be ligands of the [Fe4S4] centre. In the active state, the labile iron ion of the [Fe4S4] cluster is not coordinated by Cys but by water molecules.
The iron-responsive element-binding protein (IRE-BP) and 3-isopropylmalate dehydratase (α-isopropylmalate isomerase; EC 4.2.1.33), an enzyme catalysing the second step in the biosynthesis of leucine, are known aconitase homologues. Iron regulatory elements (IREs) constitute a family of 28-nucleotide, non-coding, stem-loop structures that regulate iron storage, heme synthesis and iron uptake. They also participate in ribosome binding and control the mRNA turnover (degradation). The specific regulator protein, the IRE-BP, binds to IREs in both 5' and 3' regions, but only to RNA in the apo form, without the Fe-S cluster. Expression of IRE-BP in cultured cells has revealed that the protein functions either as an active aconitase, when cells are iron-replete, or as an active RNA-binding protein, when cells are iron-depleted. Mutant IRE-BPs, in which any or all of the three Cys residues involved in Fe-S formation are replaced by serine, have no aconitase activity, but retain RNA-binding properties.
Aconitase is inhibited by fluoroacetate, therefore fluoroacetate is poisonous. The iron sulfur cluster is highly sensitive to oxidation by superoxide.[9]
Mechanism

Aconitase employs a dehydration-hydration mechanism.[10] The catalytic residues involved are His-101 and Ser-642.[10] His-101 protonates the hydroxyl group on C3 of citrate, allowing it to leave as water, and Ser-642 concurrently abstracts the proton on C2, forming a double bond between C2 and C3, forming a cis-aconitate intermediate.[10][13] At this point, the intermediate is rotated 180°.[10] This rotation is referred to as a "flip."[11] Because of this flip, the intermediate is said to move from a "citrate mode" to a "isocitrate mode."[14]
How exactly this flip occurs is debatable. One theory is that, in the rate-limiting step of the mechanism, the cis-aconitate is released from the enzyme, then reattached in the isocitrate mode to complete the reaction.[14] This rate-liming step ensures that the right stereochemistry, specifically (2R,3S), is formed in the final product.[14][15] Another hypothesis is that cis-aconitate stays bound to the enzyme while it flips from the citrate to the isocitrate mode.[10]
In either case, flipping cis-aconitate allows the dehydration and hydration steps to occur on opposite faces of the intermediate.[10] Aconitase catalyzes trans elimination/addition of water, and the flip guarantees that the correct stereochemistry is formed in the product.[10][11] To complete the reaction, the serine and histidine residues reverse their original catalytic actions: the histidine, now basic, abstracts a proton from water, priming it as a nucleophile to attack at C2, and the protonated serine is deprotonated by the cis-aconitate double bond to complete the hydration, producing isocitrate.[10]
Family members
Aconitases are expressed in bacteria to humans. Humans express the following two aconitase isozymes:
|
| ||||||||||||||||||||||||||||||||||||||||||||||||
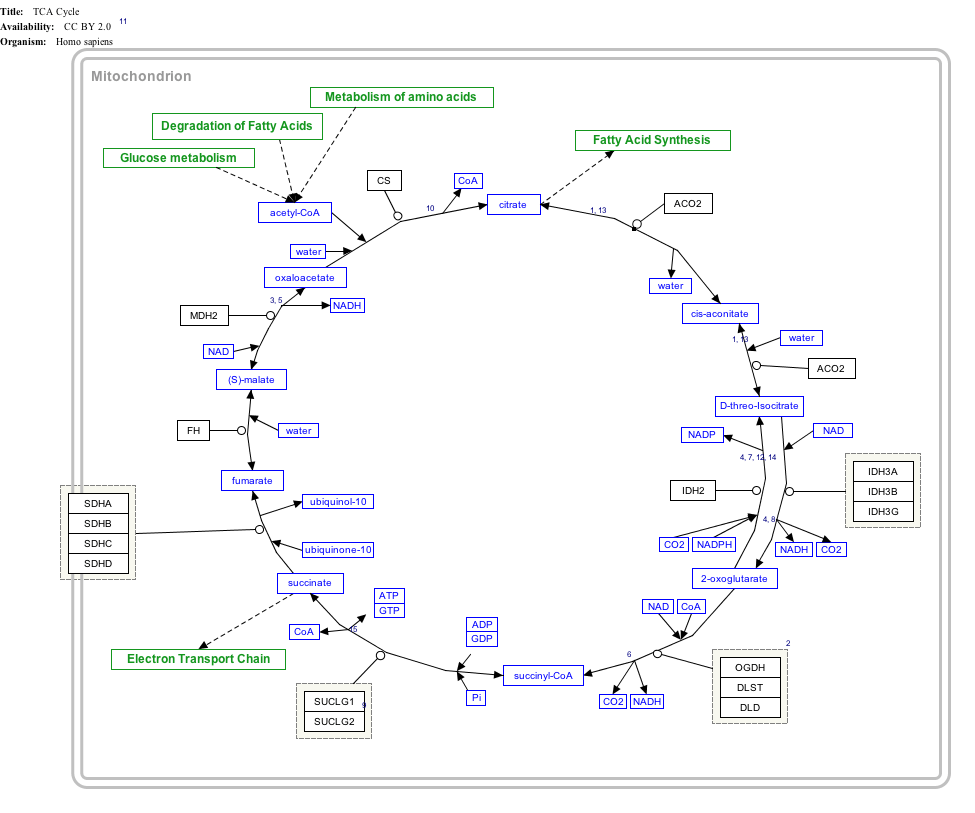
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
TCA Cycle edit
- ↑ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78".
References
- ↑ PDB: 7ACN; Lauble, H.; Kennedy, M. C.; Beinert, H.; Stout, C. D. (1992). "Crystal structures of aconitase with isocitrate and nitroisocitrate bound". Biochemistry. 31 (10): 2735–48. doi:10.1021/bi00125a014. PMID 1547214.
- ↑ PDB: 1ACO; Lauble, H; Kennedy, MC; Beinert, H; Stout, CD (1994). "Crystal Structures of Aconitase with Trans-aconitate and Nitrocitrate Bound". Journal of Molecular Biology. 237 (4): 437–51. doi:10.1006/jmbi.1994.1246. PMID 8151704.
- ↑ Beinert H, Kennedy MC (Dec 1993). "Aconitase, a two-faced protein: enzyme and iron regulatory factor". FASEB Journal. 7 (15): 1442–9. PMID 8262329.
- ↑ Flint, Dennis H.; Allen, Ronda M. (1996). "Iron−Sulfur Proteins with Nonredox Functions". Chemical Reviews. 96 (7): 2315–34. doi:10.1021/cr950041r.
- ↑ Beinert H, Kennedy MC, Stout CD (Nov 1996). "Aconitase as Ironminus signSulfur Protein, Enzyme, and Iron-Regulatory Protein". Chemical Reviews. 96 (7): 2335–2374. doi:10.1021/cr950040z. PMID 11848830.
- 1 2 3 4 Robbins AH, Stout CD (1989). "The structure of aconitase". Proteins. 5 (4): 289–312. doi:10.1002/prot.340050406. PMID 2798408.
- 1 2 3 Robbins AH, Stout CD (May 1989). "Structure of activated aconitase: formation of the [4Fe-4S] cluster in the crystal". Proceedings of the National Academy of Sciences of the United States of America. 86 (10): 3639–43. doi:10.1073/pnas.86.10.3639. PMC 287193
 . PMID 2726740.
. PMID 2726740. - ↑ Lauble H, Kennedy MC, Beinert H, Stout CD (Mar 1992). "Crystal structures of aconitase with isocitrate and nitroisocitrate bound". Biochemistry. 31 (10): 2735–48. doi:10.1021/bi00125a014. PMID 1547214.
- ↑ Gardner, Paul R. (2002). "Aconitase: Sensitive target and measure of superoxide". Superoxide Dismutase. Methods in Enzymology. 349. pp. 9–23. doi:10.1016/S0076-6879(02)49317-2. ISBN 978-0-12-182252-1.
- 1 2 3 4 5 6 7 8 9 Takusagawa F. "Chapter 16: Citric Acid Cycle" (PDF). Takusagawa’s Note. The University of Kansas. Retrieved 2011-07-10.
- 1 2 3 Beinert H, Kennedy MC, Stout CD (Nov 1996). "Aconitase as Ironminus signSulfur Protein, Enzyme, and Iron-Regulatory Protein" (PDF). Chemical Reviews. 96 (7): 2335–2374. doi:10.1021/cr950040z. PMID 11848830.
- 1 2 PDB: 1C96; Lloyd SJ, Lauble H, Prasad GS, Stout CD (December 1999). "The mechanism of aconitase: 1.8 A resolution crystal structure of the S642a:citrate complex". Protein Sci. 8 (12): 2655–62. doi:10.1110/ps.8.12.2655. PMC 2144235
 . PMID 10631981.
. PMID 10631981. - ↑ Han D, Canali R, Garcia J, Aguilera R, Gallaher TK, Cadenas E (Sep 2005). "Sites and mechanisms of aconitase inactivation by peroxynitrite: modulation by citrate and glutathione". Biochemistry. 44 (36): 11986–96. doi:10.1021/bi0509393. PMID 16142896.
- 1 2 3 Lauble H, Stout CD (May 1995). "Steric and conformational features of the aconitase mechanism". Proteins. 22 (1): 1–11. doi:10.1002/prot.340220102. PMID 7675781.
- ↑ "Aconitase family". The Prosthetic groups and Metal Ions in Protein Active Sites Database Version 2.0. The University of Leeds. 1999-02-02. Archived from the original on 8 June 2011. Retrieved 2011-07-10.
Further reading
- Frishman D, Hentze MW (Jul 1996). "Conservation of aconitase residues revealed by multiple sequence analysis. Implications for structure/function relationships". European Journal of Biochemistry / FEBS. 239 (1): 197–200. doi:10.1111/j.1432-1033.1996.0197u.x. PMID 8706708.
External links
- Aconitase at the US National Library of Medicine Medical Subject Headings (MeSH)
- Proteopedia Aconitase - the Aconitase structure in interactive 3D