Hydrosilylation
Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds.[1] Ordinarily the reaction is conducted catalytically and usually the substrates are unsaturated organic compounds. Alkenes and alkynes give alkyl and vinyl silanes; aldehydes and ketones give silyl ethers. The process was first reported in academic literature in 1947.[2] Hydrosilylation has been called the "most important application of platinum in homogeneous catalysis."[3]
Scope and mechanism
The catalytic transformation represents an important method for preparing organosilicon compounds. An idealized transformation is illustrated by the addition of triethylsilane to diphenylacetylene:[4]
- Et3SiH + PhC≡CPh → Et3Si(Ph)C=CH(Ph)
The reaction is related mechanistically to hydrogenation, and similar catalysts are sometimes employed for the two catalytic processes. Popular industrial catalysts are "Speier's catalyst," H2PtCl6, and Karstedt’s catalyst (an alkene-stabilized platinum(0) catalyst.[5] One prevalent mechanism, called the Chalk-Harrod mechanism, assumes an intermediate metal complex that contains a hydride, a silyl ligand (R3Si), and the alkene substrate. The reaction usually produces anti-Markovnikov addition alkane, i.e., silicon on the terminal carbon.[1] Variations of the Chalk-Harrod mechanism. Some cases involve insertion of alkene into M-Si bond followed by reductive elimination. In certain cases, hydrosilylation results in vinyl or allylic silanes resulting from beta-hydride elimination.[6]
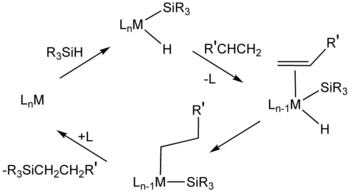
These reactions can also be catalyzed using nanomaterial-based catalysts.
Asymmetric hydrosilylation
Using chiral phosphines as spectator ligands, catalysts have been developed for catalytic asymmetric hydrosilation. A well studied reaction is the addition of trichlorosilane to styrene to give 1-phenyl-1-(trichlorosilyl)ethane:
- Cl3SiH + PhCHCH2 → (Ph)(CH3)CHSiCl3
Nearly perfect enantioselectivities (ee's) can be achieved using palladium catalysts supported by binaphthyl-substituted monophosphine ligands.[7]
Surface hydrosilylation
Silicon wafers can be etched in hydrofluoric acid (HF) to remove the native oxide and form a hydrogen-terminated silicon surface. The hydrogen-terminated surfaces undergo hydrosilation with unsaturated compounds (such as terminal alkenes and alkynes), to form a stable monolayer on the surface. For example:
- Si-H + H2C=CH(CH2)7CH3 → Si-CH2CHH-(CH2)7CH3
The hydrosilylation reaction can be initiated with UV light at room temperature or with heat (typical reaction temperature 120-200 °C), under moisture- and oxygen-free conditions.[8] The resulting monolayer, which is stable and inert, inhibits oxidation of the base silicon layer, relevant to various device applications.[9]
References
- 1 2 "Hydrosilylation A Comprehensive Review on Recent Advances" B. Marciniec (ed.), Advances in Silicon Science, Springer Science, 2009. doi:10.1007/978-1-4020-8172-9
- ↑ Sommer, L.; Pietrusza, E.; Whitmore, F. "Peroxide-catalyzed addition of trichlorosilane to 1-octene". J. Amer. Chem. Soc. 69 (1): 188. doi:10.1021/ja01193a508.
- ↑ Renner, H.; Schlamp, G.; Kleinwächter, I.; Drost, E.; Lüschow, H. M.; Tews, P.; Panster, P.; Diehl, M.; Lang, J.; Kreuzer, T.; Knödler, A.; Starz, K. A.; Dermann, K.; Rothaut, J.; Drieselman, R. (2002). "Platinum group metals and compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a21_075.
- ↑ James L. Fry, Ronald J. Rahaim Jr., Robert E. Maleczka Jr. "Triethylsilane", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, 2007. doi:10.1002/047084289X.rt226.pub2
- ↑ C. Elschenbroich, Organometallics (2006) Wiley and Sons-VCH: Weinheim. ISBN 978-3-527-29390-2
- ↑ Troegel, D.; Stohrer, J., "Recent advances and actual challenges in late transition metal catalyzed hydrosilylation of olefins from an industrial point of view", Coord. Chem. Rev. 2011, 255, 1440-1459. doi:10.1016/j.ccr.2010.12.025
- ↑ Hayashi, T.; Yamasaki, K. (2007). "C–E Bond Formation through Asymmetric Hydrosilylation of Alkenes". In Crabtree, Robert H.; Mingos, D. Michael P. Comprehensive Organometallic Chemistry III. Amsterdam: Elsevier. doi:10.1016/B0-08-045047-4/00140-0. ISBN 978-0-08-045047-6.
- ↑ "Photoreactivity of Unsaturated Compounds with Hydrogen-Terminated Silicon (111)," R. L. Cicero, M. R. Linford, C. E. D. Chidsey, Langmuir 16, 5688-5695 (2000).
- ↑ "Direct electrical detection of DNA Hybridization at DNA-modified silicon surfaces," W.Cai, J. Peck, D. van der Weide, and R.J. Hamers, Biosensors and Bioelectronics 19, 1013-1019 (2004).
Further reading
- Applied homogeneous catalysis with organometallic compounds : a comprehensive handbook : applications, developments. Boy Cornils; W A Herrmann. Publisher: Weinheim ; New York : Wiley-VCH, ©2000.
- Comprehensive handbook on hydrosilylation. Bogdan Marciniec. Publisher: Oxford [u.a.] : Pergamon Press, 1992.
- Rhodium complexes as hydrosilylation catalysts. N.K. Skvortsov. // Rhodium Express. 1994. No 4 (May). P. 3 - 36 (Eng). ISSN 0869-7876
Additional specialized literature
- "Alkyl Monolayers on Silicon Prepared from 1-Alkenes and Hydrogen-Terminated Silicon," M. R. Linford, P. Fenter, P. M. Eisenberger and C. E. D. Chidsey, J. Am. Chem. Soc. 117, 3145-3155 (1995).
- "Synthesis and characterization of DNA-modified Si(111) Surfaces," T. Strother, W. CAi, X. Zhao, R.J. Hamers, and L.M. Smith, J. Am. Chem. Soc. 122, 1205-1209 (2000).
- "T. Strother, R.J. Hamers, and L.M. Smith, "Surface Chemistry of DNA Covalent Attachment to the Silicon(100) Surface". Langmuir, 2002, 18, 788-796.
- "Covalently Modified Silicon and Diamond Surfaces: Resistance to Non-Specific Protein Adsorption and Optimization for Biosensing," T.L. Lasseter, B.H. Clare, N.L. Abbott, and R.J. Hamers. J. Am. Chem. Soc. 2004, 126, 10220-10221.