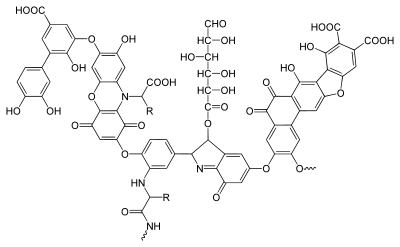
Humic acid
Humic acids are a principal component of humic substances, which are the major organic constituents of soil (humus), peat and coal. It is also a major organic constituent of many upland streams, dystrophic lakes, and ocean water.[1] It is produced by biodegradation of dead organic matter. It is not a single acid; rather, it is a complex mixture of many different acids containing carboxyl and phenolate groups so that the mixture behaves functionally as a dibasic acid or, occasionally, as a tribasic acid. Humic acids can form complexes with ions that are commonly found in the environment creating humic colloids. Humic acids are insoluble in water at acid pH, whereas fulvic acids are also derived from humic substances but are soluble in water across the full range of pH.[2] Humic and fulvic acids are commonly used as a soil supplement in agriculture, and less commonly as a human nutritional supplement. As a nutrition supplement, fulvic acid can be found in a liquid form as a component of mineral colloids. Fulvic acids are poly-electrolytes and are unique colloids that diffuse easily through membranes whereas all other colloids do not.[3]
Formation and description
The formation of humic substances is one of the least understood aspects of humus chemistry and one of the most intriguing. There are three main theories to explain it: the lignin theory of Waksman (1932), the polyphenol theory, and the sugar-amine condensation theory of Maillard (1911).[4][5] Humic substances are formed by the microbial degradation of dead plant matter, such as lignin and charcoal.[6][7] Humic substances are very resistant to further biodegradation. The precise properties and structure of a given sample depend on the water or soil source and the specific conditions of extraction. Nevertheless, the average properties of humic substances from different sources are remarkably similar.
Humic substances in soils and sediments can be divided into three main fractions: humic acids, fulvic acids, and humin. The humic and fulvic acids are extracted as a colloidal sol from soil and other solid phase sources into a strongly basic aqueous solution of sodium hydroxide or potassium hydroxide. Humic acids are precipitated from this solution by adjusting the pH to 1 with hydrochloric acid, leaving the fulvic acids in solution. This is the operational distinction between humic and fulvic acids. Humin is insoluble in dilute alkali. The alcohol-soluble portion of the humic fraction is, in general, named ulmic acid. So-called "gray humic acids" (GHA) are soluble in low-ionic-strength alkaline media; "brown humic acids" (BHA) are soluble in alkaline conditions independent of ionic strength; and fulvic acids (FA) are soluble independent of pH and ionic strength.[8]
Liquid chromatography and liquid-liquid extraction can be used to separate the components that make up a humic substance. Substances identified include mono-, di-, and tri-hydroxy acids, fatty acids, dicarboxylic acids, linear alcohols, phenolic acids, and terpenoids.[9]
Criticism
Decomposition products of dead plant materials form intimate associations with minerals, making it difficult to isolate and characterize soil organic constituents. 18th century soil chemists successfully used alkaline extraction to isolate a portion of the organic constituents in soil. This led to the theory that a 'humification' process created 'humic substances': most commonly 'humic acid', 'fulvic acid', and 'humin'.[10] However, these humic substances have not been observed in soil. Although 'humification' theory is unsupported by evidence, "the underlying theory persists in the contemporary literature, including current textbooks."[11] Attempts to redefine 'humic substances' in valid terms have resulted in a proliferation of incompatible definitions, "with far-reaching implications beyond our ability to communicate scientifically accurate soil processes and properties."[12]
Chemical characteristics of humic substances
A typical humic substance is a mixture of many molecules, some of which are based on a motif of aromatic nuclei with phenolic and carboxylic substituents, linked together; the illustration shows a typical structure. The functional groups that contribute most to surface charge and reactivity of humic substances are phenolic and carboxylic groups.[1] Humic acids behave as mixtures of dibasic acids, with a pK1 value around 4 for protonation of carboxyl groups and around 8 for protonation of phenolate groups. There is considerable overall similarity among individual humic acids.[13] For this reason, measured pK values for a given sample are average values relating to the constituent species. The other important characteristic is charge density. The molecules may form a supramolecular structure held together by non-covalent forces, such as Van der Waals force, π-π, and CH-π bonds.[14]
The presence of carboxylate and phenolate groups gives the humic acids the ability to form complexes with ions such as Mg2+, Ca2+, Fe2+ and Fe3+. Many humic acids have two or more of these groups arranged so as to enable the formation of chelate complexes.[15] The formation of (chelate) complexes is an important aspect of the biological role of humic acids in regulating bioavailability of metal ions.[13]
Determination of humic acids in water samples
The presence of humic acid in water intended for potable or industrial use can have a significant impact on the treatability of that water and the success of chemical disinfection processes. Accurate methods of establishing humic acid concentrations are therefore essential in maintaining water supplies, especially from upland peaty catchments in temperate climates.
As a lot of different bio-organic molecules in very diverse physical associations are mixed together in natural environments, it is cumbersome to measure their exact concentrations in the humic superstructure. For this reason, concentrations of humic acid are traditionally estimated out of concentrations of organic matter (typically from concentrations of total organic carbon (TOC) or dissolved organic carbon (DOC).
Extraction procedures are bound to alter some of the chemical linkages present in the soil humic substances (mainly ester bonds in biopolyesters such as cutins and suberins). The humic extracts are composed of large numbers of different bio-organic molecules that have not yet been totally separated and identified. However, single classes of residual biomolecules have been identified by selective extractions and chemical fractionation, and are represented by alkanoic and hydroxy alkanoic acids, resins, waxes, lignin residues, sugars, and peptides.
Health issues
Humic and fulvic acids, when present in treated drinking water, can react with the chemicals used in the chlorination process to form disinfection byproducts such as dihaloacetonitriles, which are toxic to humans.[16][17]
Ecological effects
Organic matter soil amendments have been known by farmers to be beneficial to plant growth for longer than recorded history.[18] However, the chemistry and function of the organic matter have been a subject of controversy since humans began their postulating about it in the 18th century. Until the time of Liebig, it was supposed that humus was used directly by plants, but, after Liebig had shown that plant growth depends upon inorganic compounds, many soil scientists held the view that organic matter was useful for fertility only as it was broken down with the release of its constituent nutrient elements into inorganic forms. At the present time, soil scientists hold a more holistic view and at least recognize that humus influences soil fertility through its effect on the water-holding capacity of the soil. Also, since plants have been shown to absorb and translocate the complex organic molecules of systemic insecticides, they can no longer discredit the idea that plants may be able to absorb the soluble forms of humus;[19] this may in fact be an essential process for the uptake of otherwise insoluble iron oxides.
A study on the effects of humic acid on plant growth was conducted at Ohio State University which said in part “humic acids increased plant growth” and that there were “relatively large responses at low application rates” [20]
A 1998 study by scientists at the North Carolina State University College of Agriculture and Life Sciences showed that addition of humate to soil significantly increased root mass in creeping bentgrass turf.[21][22]
Ancient masonry
In Ancient Egypt, according to archeology, straw was mixed with mud in order to produce building bricks. Straw produces stronger bricks that are less likely to break or lose their shape. Modern investigations have found that humic acid is released from straw when mixed with mud, basically a mixture of sand and clay. Humic acid increases clay's plasticity.[23]
See also
References
- 1 2 3 Stevenson F.J. (1994). Humus Chemistry: Genesis, Composition, Reactions. New York: John Wiley & Sons.
- ↑ MacCarthy, Patrick (November 2001). "The Principles of Humic Substances". Soil Science. 166 (11): 738–751. doi:10.1097/00010694-200111000-00003.
- ↑ Yamauchi, Masashige; Katayama, Sadamu; Todoroki, Toshiharu; Watanable, Toshio (1984). "Total synthesis of fulvic acid". Journal of the Chemical Society, Chemical Communications (23): 1565–6. doi:10.1039/C39840001565.
Synthesis of fulvic acid (1a) was accomplished by a route involving selective ozonization of 9-propenylpyranobenzopyran (1c), obtained by a regioselective cyclization of the 2-methylsulphinylmethyl 1,3-dione(3c)
- ↑ Stevenson, F.J. (1994). Humus Chemistry: Genesis, Composition, Reactions, Wiley & Sons, New York, 1994, pp. 188-210. ISBN 0471594741.
- ↑ Tan, K. H. (2014). Humic matter in soil and the environment: principles and controversies. 2nd ed. Boca Ranton: CRC Press. ISBN 1482234459.
- ↑ Ponomarenko, E.V.; Anderson, D.W. (2001), "Importance of charred organic matter in Black Chernozem soils of Saskatchewan", Canadian Journal of Soil Science, 81 (3): 285–297,
The present paradigm views humus as a system of heteropolycondensates, largely produced by the soil microflora, in varying associations with clay (Anderson 1979). Because this conceptual model, and simulation models rooted within the concept, do not accommodate a large char component, a considerable change in conceptual understanding (a paradigm shift) appears imminent.
- ↑ Mao, J.-D.; Johnson, R. L.; Lehmann, J.; Olk, J.; Neeves, E. G.; Thompson, M. L.; Schmidt-Rohr, K. (2012). "Abundant and stable char residues in soils: implications for soil fertility and carbon sequestration". Environmental Science and Technology. 46: 9571–9576. doi:10.1021/es301107c.
- ↑ Baigorri R; Fuentes M; González-Gaitano G; García-Mina JM; Almendros G; González-Vila FJ. (2009). "Complementary Multianalytical Approach To Study the Distinctive Structural Features of the Main Humic Fractions in Solution: Gray Humic Acid, Brown Humic Acid, and Fulvic Acid". J Agric Food Chem. 57 (8): 3266–72. doi:10.1021/jf8035353. PMID 19281175.
- ↑ Fiorentino G., Spaccini R., Piccolo A (2006). "Separation of molecular constituents from a humic acid by solid-phase extraction following a transesterification reaction". Talanta. 68 (4): 1135–1142. doi:10.1016/j.talanta.2005.07.037. PMID 18970442.
- ↑ Lehmann, J.; Kleber, M. (2015-12-03), "The contentious nature of soil organic matter", Nature, 528, doi:10.1038/nature16069,
The development of this extraction method preceded theory, tempting scientists to develop explanations for the synthesis of materials resembling operationally extracted ‘humic substances’, rather than to develop an understanding of the nature of all organic matter in soil.
- ↑ Lehmann, J.; Kleber, M. (2015-12-03), "The contentious nature of soil organic matter", Nature, 528, doi:10.1038/nature16069,
This lack of evidence means that 'humification' is increasingly questioned, yet the underlying theory persists in the contemporary literature, including current textbooks.
- ↑ Lehmann, J.; Kleber, M. (2015-12-03), "The contentious nature of soil organic matter", Nature, 528, doi:10.1038/nature16069,
The issue has also been approached by redefining ‘humic substances’ as the portion of soil organic matter that cannot be molecularly characterized or by calling all soil organic matter ‘humus’. We argue that this compromise - maintaining terminology but altering its meanings in varying ways — hampers scientific progress beyond the soil sciences.The [need for accurate models] of soil organic matter does not allow a confusing middle path; it requires leaving the traditional view behind to bring about lasting innovation and progress. This is critical as scientific fields outside the soil sciences base their research on the false premise of the existence of ‘humic substances’. Thus an issue of terminology becomes a problem of false inference, with far-reaching implications beyond our ability to communicate scientifically accurate soil processes and properties.
- 1 2 Ghabbour, E.A.; Davies, G. (Editors) (2001). Humic Substances: Structures, Models and Functions. Cambridge, U.K.: RSC publishing. ISBN 978-0-85404-811-3.
- ↑ Piccolo, A. (2002). "The Supramolecular structure of humic substances. A novel understanding of humus chemistry and implications in soil science". Advances in Agronomy. Advances in Agronomy. 75: 57–134. doi:10.1016/S0065-2113(02)75003-7. ISBN 978-0-12-000793-6.
- ↑ Tipping, E (1994). "'WHAM – a chemical equilibrium model and computer code for waters, sediments, and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances". Computers and Geosciences. 20 (6): 973–1023. Bibcode:1994CG.....20..973T. doi:10.1016/0098-3004(94)90038-8.
- ↑ Oliver, Barry G. (1983). "Dihaloacetonitriles in drinking water: Algae and fulvic acid as precursors". Environmental Science & Technology. 17 (2): 80. Bibcode:1983EnST...17...80O. doi:10.1021/es00108a003.
- ↑ Peters, Ruud J.B.; De Leer, Ed W.B.; De Galan, Leo (1990). "Dihaloacetonitriles in Dutch drinking waters". Water Research. 24 (6): 797. doi:10.1016/0043-1354(90)90038-8.
- ↑ Lapedes, Daniel N., ed. (1966). McGraw-Hill encyclopedia of science and technology: an international reference work, Volume 12. McGraw-Hill. p. 428. ISBN 0070452652.
The value of adding organic matter to the soil in the form of animal manures, green manures, and crop residues for producing favorable soil tilth has been known since ancient times
- ↑ Pan American Union. Dept. of Cultural Affairs. División de Fomento Científico, Pan American Union. Dept. of Scientific Affairs, Organization of American States. Dept. of Scientific Affairs (1984). Ciencia interamericana: Volumes 24–27.
And since plants have shown their ability to absorb and translocate the complex molecules of systemic insecticides, they can no longer discredit the idea that plants are able to absorb the soluble humic nutrients, containing by far ...
- ↑ Arancon, Norman Q.; Edwards, Clive. A.; Lee, Stephen; Byrne, Robert (2006). "Effects of humic acids from vermicomposts on plant growth" (PDF). European Journal of Soil Biology. 42: S65. doi:10.1016/j.ejsobi.2006.06.004.
- ↑ Cooper, R. J.; Liu, Chunhua; Fisher, D. S. (1998). "Influence of Humic Substances on Rooting and Nutrient Content of Creeping Bentgrass". Crop Science. 38 (6): 1639. doi:10.2135/cropsci1998.0011183X003800060037x.
- ↑ Liu, Chunhua; Cooper, R. J. (August 1999). "Humic Substances Their Influence on Creeping Bentgrass Growth and Stress Tolerance" (PDF). TurfGrass Trends: 6.
- ↑ Lucas, A.; Harris, J.R. (1998). Ancient Egyptian Materials and Industries. New York: Dover Publications. p. 49. ISBN 0-486-40446-3.
Further reading
- Hessen, D.O.; Tranvik, L.J. (Editors) (1998). Aquatic humic substances: ecology and biogeochemistry. Berlin: Springer. ISBN 3-540-63910-1.
- Sillanpää, M. (Ed.) Natural Organic Matter in Water, Characterization and Treatment Methods ISBN 9780128015032