Vinyl halide
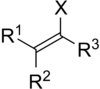
In organic chemistry, a vinyl halide is any alkene with at least one halide substituent bonded directly on one of the alkene carbons. Vinyl chloride is one such substance.
Vinyl halides are very useful synthetic intermediates due to the vast number of reactions that make use of them. These include conversion to vinyl Grignard reagents, elimination to give the corresponding alkyne, and most importantly their use in cross-coupling reactions (e.g. Suzuki-Miyaura coupling, Stille coupling, Heck coupling, etc.).
As a result, there is a large number of reactions to form vinyl halides, which includes the reaction of vinyl organometallic species with halogens, and the Takai and Wittig olefination reactions. Olefin metathesis has also been shown to offer a distinct synthesis approach to access vinyl halides efficiently and stereoselectively.[1]
Besides, some vinyl halides are useful for synthesizing polymers and copolymers, see e.g. polyvinyl chloride or polyvinyl fluoride. The unsubstituted vinyl halides (R1 = R2 = R3 = H) may polymerize spontaneously under certain conditions.
References
- ↑ Koh, Ming Joo; Nguyen, Thach T.; Zhang, Hanmo; Schrock, Richard R.; Hoveyda, Amir H. "Direct synthesis of Z-alkenyl halides through catalytic cross-metathesis". Nature. 531 (7595): 459–465. doi:10.1038/nature17396.
- ↑ Nguyen, Thach T.; Koh, Ming Joo; Shen, Xiao; Romiti, Filippo; Schrock, Richard R.; Hoveyda, Amir H. (2016-04-29). "Kinetically controlled E-selective catalytic olefin metathesis". Science. 352 (6285): 569–575. doi:10.1126/science.aaf4622.