Grain boundary

A grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are 2D defects in the crystal structure, and tend to decrease the electrical and thermal conductivity of the material. Most grain boundaries are preferred sites for the onset of corrosion and for the precipitation of new phases from the solid. They are also important to many of the mechanisms of creep. On the other hand, grain boundaries disrupt the motion of dislocations through a material, so reducing crystallite size is a common way to improve mechanical strength, as described by the Hall–Petch relationship.
High and low angle boundaries
It is convenient to separate grain boundaries by the extent of the mis-orientation between the two grains. Low angle grain boundaries (LAGBs) or subgrain boundaries are those with a misorientation less than about 15 degrees. Generally speaking they are composed of an array of dislocations and their properties and structure are a function of the misorientation. In contrast the properties of high angle grain boundaries (HAGBs), whose misorientation is greater than about 11 degrees (the transition angle varies from 10–15 degrees depending on the material), are normally found to be independent of the misorientation. However, there are 'special boundaries' at particular orientations whose interfacial energies are notably lower than those of general HAGBs.

The simplest boundary is that of a tilt boundary where the rotation axis is parallel to the boundary plane. This boundary can be conceived as forming from a single, contiguous crystallite or grain which is gradually bent by some external force. The energy associated with the elastic bending of the lattice can be reduced by inserting a dislocation, which is essentially a half-plane of atoms that act like a wedge, that creates a permanent misorientation between the two sides. As the grain is bent further, more and more dislocations must be introduced to accommodate the deformation resulting in a growing wall of dislocations – a low-angle boundary. The grain can now be considered to have split into two sub-grains of related crystallography but notably different orientations.
An alternative is a twist boundary where the mis-orientation occurs around an axis that is perpendicular to the boundary plane. This type of boundary incorporates two sets of screw dislocations. If the Burgers vectors of the dislocations are orthogonal, then the dislocations do not strongly interact and form a square network. In other cases, the dislocations may interact to form a more complex hexagonal structure.
These concepts of tilt and twist boundaries represent somewhat idealized cases. The majority of boundaries are of a mixed type, containing dislocations of different types and Burgers vectors, in order to create the best fit between the neighboring grains.
If the dislocations in the boundary remain isolated and distinct, the boundary can be considered to be low-angle. If deformation continues, the density of dislocations will increase and so reduce the spacing between neighboring dislocations. Eventually, the cores of the dislocations will begin to overlap and the ordered nature of the boundary will begin to break down. At this point the boundary can be considered to be high-angle and the original grain to have separated into two entirely separate grains.
In comparison to LAGBs, high-angle boundaries are considerably more disordered, with large areas of poor fit and a comparatively open structure. Indeed, they were originally thought to be some form of amorphous or even liquid layer between the grains. However, this model could not explain the observed strength of grain boundaries and, after the invention of electron microscopy, direct evidence of the grain structure meant the hypothesis had to be discarded. It is now accepted that a boundary consists of structural units which depend on both the misorientation of the two grains and the plane of the interface. The types of structural unit that exist can be related to the concept of the coincidence site lattice, in which repeated units are formed from points where the two misoriented lattices happen to coincide.
In coincident site lattice (CSL) theory, the degree of fit (Σ) between the structures of the two grains is described by the reciprocal of the ratio of coincidence sites to the total number of sites. In this framework, it is possible to draw the lattice for the 2 grains and count the number of atoms that are shared (coincidence sites), and the total number of atoms on the boundary (total number of site). For example, when Σ=3 there will be one atom each 3 that will be shared between the two lattices. Thus a boundary with high Σ might be expected to have a higher energy than one with low Σ. Low-angle boundaries, where the distortion is entirely accommodated by dislocations, are Σ1. Some other low-Σ boundaries have special properties, especially when the boundary plane is one that contains a high density of coincident sites. Examples include coherent twin boundaries (Σ3) and high-mobility boundaries in FCC materials (Σ7). Deviations from the ideal CSL orientation may be accommodated by local atomic relaxation or the inclusion of dislocations into the boundary.
Describing a boundary
A boundary can be described by the orientation of the boundary to the two grains and the 3-D rotation required to bring the grains into coincidence. Thus a boundary has 5 macroscopic degrees of freedom. However, it is common to describe a boundary only as the orientation relationship of the neighbouring grains. Generally, the convenience of ignoring the boundary plane orientation, which is very difficult to determine, outweighs the reduced information. The relative orientation of the two grains is described using the rotation matrix:
Using this system the rotation angle θ is:
while the direction [uvw] of the rotation axis is:
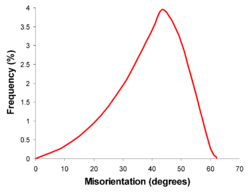
The nature of the crystallography involved limits the misorientation of the boundary. A completely random polycrystal, with no texture, thus has a characteristic distribution of boundary misorientations (see figure). However, such cases are rare and most materials will deviate from this ideal to a greater or lesser degree.
Boundary energy

The energy of a low-angle boundary is dependent on the degree of misorientation between the neighbouring grains up to the transition to high-angle status. In the case of simple tilt boundaries the energy of a boundary made up of dislocations with Burgers vector b and spacing h is predicted by the Read-Shockley equation:
where:
with is the shear modulus, is Poisson's ratio, and is the radius of the dislocation core. It can be seen that as the energy of the boundary increases the energy per dislocation decreases. Thus there is a driving force to produce fewer, more misoriented boundaries (i.e., grain growth).
The situation in high-angle boundaries is more complex. Although theory predicts that the energy will be a minimum for ideal CSL configurations, with deviations requiring dislocations and other energetic features, empirical measurements suggest the relationship is more complicated. Some predicted troughs in energy are found as expected while others missing or substantially reduced. Surveys of the available experimental data have indicated that simple relationships such as low are misleading:
It is concluded that no general and useful criterion for low energy can be enshrined in a simple geometric framework. Any understanding of the variations of interfacial energy must take account of the atomic structure and the details of the bonding at the interface.[1]
Excess volume
The excess volume is another important property in the characterization of grain boundaries. It describes how much expansion is induced by the presence of a GB and is thought that the degree and susceptibility of segregation is directly proportional to this. Despite the name the excess volume is actually a change in length, this is because of the 2D nature of GBs the length of interest is the expansion normal to the GB plane. Although a rough linear relationship between GB energy and excess volume exists the orientations where this relationship is violated can behave significantly differently affecting mechanical and electrical properties.[2]
Experimental techniques have been developed which directly probe the excess volume and have been used to explore the properties of nanocrystalline copper and nickel.[3][4] Theoretical methods have also been developed [5] and are in good agreement. A key observation is that there is an inverse relationship with the bulk modulus meaning that the larger the bulk modulus (the ability to compress a material) the smaller the excess volume will be, there is also direct relationship with the lattice constant, this provides methodology to find materials with a desirable excess volume for a specific application.
Boundary migration
The movement of grain boundaries (HAGB) has implications for recrystallization and grain growth while subgrain boundary (LAGB) movement strongly influences recovery and the nucleation of recrystallization.
A boundary moves due to a pressure acting on it. It is generally assumed that the velocity is directly proportional to the pressure with the constant of proportionality being the mobility of the boundary. The mobility is strongly temperature dependent and often follows an Arrhenius type relationship:
The apparent activation energy (Q) may be related to the thermally activated atomistic processes that occur during boundary movement. However, there are several proposed mechanisms where the mobility will depend on the driving pressure and the assumed proportionality may break down.
It is generally accepted that the mobility of low-angle boundaries is much lower than that of high-angle boundaries. The following observations appear to hold true over a range of conditions:
- The mobility of low-angle boundaries is proportional to the pressure acting on it.
- The rate controlling process is that of bulk diffusion
- The boundary mobility increases with misorientation.
Since low-angle boundaries are composed of arrays of dislocations and their movement may be related to dislocation theory. The most likely mechanism, given the experimental data, is that of dislocation climb, rate limited by the diffusion of solute in the bulk.[6]
The movement of high-angle boundaries occurs by the transfer of atoms between the neighbouring grains. The ease with which this can occur will depend on the structure of the boundary, itself dependent on the crystallography of the grains involved, impurity atoms and the temperature. It is possible that some form of diffusionless mechanism (akin to diffusionless phase transformations such as martensite) may operate in certain conditions. Some defects in the boundary, such as steps and ledges, may also offer alternative mechanisms for atomic transfer.

Since a high-angle boundary is imperfectly packed compared to the normal lattice it has some amount of free space or free volume where solute atoms may possess a lower energy. As a result, a boundary may be associated with a solute atmosphere that will retard its movement. Only at higher velocities will the boundary be able to break free of its atmosphere and resume normal motion.
Both low- and high-angle boundaries are retarded by the presence of particles via the so-called Zener pinning effect. This effect is often exploited in commercial alloys to minimise or prevent recrystallization or grain growth during heat-treatment.
Complexion
Grain boundaries are the preferential site for segregation of impurities, which may form a thin layer with a different composition from the bulk. For example, a thin layer of silica, which also contains impurity cations, is often present in silicon nitride. These grain boundary phases are thermodynamically stable and can be considered as quasi-two-dimensional phase, which may undergo to transition, similar to those of bulk phases. In this case structure and chemistry abrupt changes are possible at a critical value of a thermodynamic parameter like temperature or pressure.[7] This may strongly affect the macroscopic properties of the material, for example the electrical resistance or creep rates.[8] Grain boundaries can be analyzed using equilibrium thermodynamics but cannot be considered as phases, because they do not satisfy Gibbs'definition: they are inomogeneous, may have a gradient of structure, composition or properties. For this reasons they are defined as complexion: an interfacial material or stata that is in thermodynamic equilibrium with its abutting phases, with a finite and stable thickness (that is typically 2–20 Å). A complexion need the abutting phase to exist and its composition and structure need to be different from the abutting phase. Contrary to bulk phases, complexions also depend on the abutting phase. For example, silica rich amorphous layer present in Si3N3, is about 10 Å thick, but for special boundaries this equilibrium thickness is zero.[9] Complexion can be grouped in 6 categories, according to their thickness: monolayer, bilayer, trilayer, nanolayer (with equilibrium thickness between 1 and 2 nm) and wetting. In the first cases the thickness of the layer will be constant; if extra material is present it will segregate at multiple grain junction, while in the last case there is no equilibrium thickness and this is determined by the amount of secondary phase present in the material. One example of grain boundary complexion transition is the passage from dry boundary to biltilayer in Au-doped Si, which is produced by the increase of Au.[10]
See also
| Wikimedia Commons has media related to Grain boundary. |
References
- ↑ Sutton.
- ↑ Wolf D (1989). "Correlation between energy and volume expansion for grain boundaries in FCC metals". Scripta Metallurgica. 23 (11): 1913–1918. doi:10.1016/0036-9748(89)90482-1.
- ↑ E.M. Steyskal, B. Oberdorfer, W. Sprengel, M. Zehetbauer, R. Pippan, R. Würschum Direct experimental determination of grain boundary excess volume in metals Phys. Rev. Lett., 108 (5) (2012), p. 055504
- ↑ Oberdorfer B.; Setman D.; Steyskal E.M.; Hohenwarter A.; Sprengel W.; Zehetbauer M.; Pippan R.; Würschum R. (2014). "Grain boundary excess volume and defect annealing of copper after high-pressure torsion". Acta Mater. 68 (100): 189–195. doi:10.1016/j.actamat.2013.12.036.
- ↑ Bean Jonathan J.; McKenna Keith P. (2016). "Origin of differences in the excess volume of copper and nickel grain boundaries". Acta Materialia. 110: 246–257. doi:10.1016/j.actamat.2016.02.040.
- ↑ Humphreys.
- ↑ Sutton AP, Balluffi RW. (1995) Interfaces in crystalline materials. Oxford: Oxford Scientific Publications.
- ↑ Hart EW (1972). The nature and behavior of grain boundaries. New York: Plenum; p. 155.
- ↑ Cantwell P.R. et al, (2014) Grain boundary complexions. Acta Materialia 62 p.1–48
- ↑ Ma S. et al. Scripta Mater (2012) n66, p203.
Bibliography
- FJ Humphreys; M Hatherly (2004). Recrystallisation and related anealing phenomena. Elsevier.
- AP Sutton; RW Balluffi (1987). "Overview no. 61: On geometric criteria for low interfacial energy". Acta Metallurgica. 35 (9): 2177–2201. doi:10.1016/0001-6160(87)90067-8.
Further reading
- RD Doherty; DA Hughes; FJ Humphreys; JJ Jonas; D Juul Jenson; et al. (1997). "Current Issues In Recrystallisation: A Review". Materials Science and Engineering A. 238 (2): 219–274. doi:10.1016/S0921-5093(97)00424-3.
- G Gottstein; LS Shvindlerman (2009). Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications, 2nd Edition. CRC Press.