Glutamine—fructose-6-phosphate transaminase (isomerizing)
In enzymology, a glutamine-fructose-6-phosphate transaminase (isomerizing) (EC 2.6.1.16) is an enzyme that catalyzes the chemical reaction:
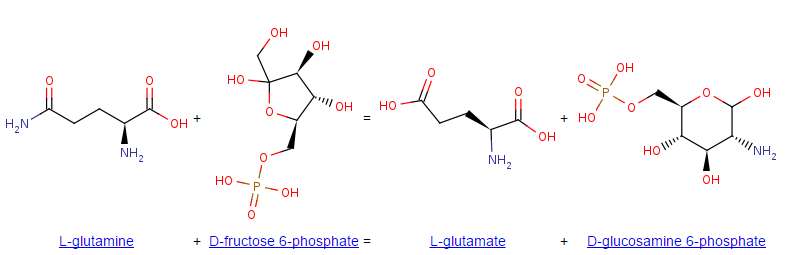
Thus, the two substrates of this enzyme are L-glutamine and D-fructose 6-phosphate, whereas its two products are L-glutamate and D-glucosamine 6-phosphate. Although the overall reaction is that of a transferase, the mechanism involves the formation of ketimine between fructose 6-phosphate and a 6-amino group from a lysine residue at the active site, which is subsequently displaced by ammonia (transamidination).
Enzyme Family
This enzyme belongs to the family of transferases, specifically the transaminases, which transfer nitrogenous groups. The systematic name of this enzyme class is L-glutamine:D-fructose-6-phosphate isomerase (deaminating). Other names in common use include: D-fructose-6-phosphate amidotransferase.
- GlcN6P synthase.
- Glucosamine-6-phosphate isomerase (glutamine-forming).
- Glucosamine-6-phosphate synthase.
- Hexosephosphate aminotransferase.
- L-glutamine-D-fructose-6-phosphate amidotransferase.
This enzyme participates in glutamate metabolism and aminosugars metabolism.
The Hexosamine Biosynthesis Pathway
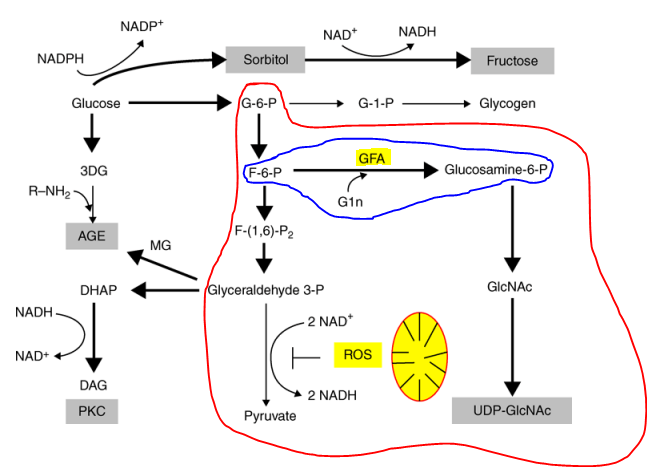
The HBP is a glucose metabolic pathway, usually accounting for only 2–5% of total glucose metabolism that has been associated with posttranslational protein modification by glycosylation and the synthesis of glycolipids, proteoglycans, and glycosylphosphatidylinositol anchors. Glucosamine-6-phosphate (GlcN-6-P) synthase is an ubiquitous enzyme catalysing the first committed step in this biosynthetic pathway leading to the formation of an activated form of N-acetyl-D-glucosamine, namely uridine 5′-diphospho-N-acetyl-D-glucosamine (UDP-GlcNAc). This sugar nucleotide provides N-acetyl-D-glucosamine for biosynthesis of a number of hexosamine-containing biomacromolecules, including bacterial peptidoglycan, fungal chitin and mannoproteins and mammalian glycoproteins and mucopolysaccharides. GlcN-6-P synthase catalyses the initial step of this pathway, it is therefore an important point of metabolic control of amino sugar biosynthesis. Moreover, this enzyme is of interest as a potential drug target for the treatment of non-insulin-dependent diabetes mellitus 1 and gastric disorders2 as well as for anti-fungal chemotherapy.
Uridine diphosphate N-acetylglucosamine
Uridine diphosphate N-acetylglucosamine or UDP-GlcNAc is a nucleotide sugar and a coenzyme in metabolism. It is used by glycosyltransferases to transfer N-acetylglucosamine residues to substrates. UDP-GlcNAc is the end product of the HBP and is then used for making glycosaminoglycans, proteoglycans, and glycolipids. UDP-GlcNAc is extensively involved in intracellular signaling as a substrate for O-linked N-acetylglucosamine transferases (OGTs) in a wide range of species. It is also involved in nuclear pore formation and nuclear signalling. OGTs and OG-ases play an important role in the structure of the cytoskeleton. In mammals, there is enrichment of OGT transcripts in the pancreas beta-cells, and UDP-GlcNAc is thought to be part of the glucose sensing mechanism. There is also evidence that it plays a part in insulin sensitivity in other cells. In plants, it is involved in the control of gibberellin production.
Cells from all three domains of life use N-acetyl-d-glucosamine (GlcNAc)1 in their cell walls, extracellular matrices, protein post-translational modifications or glycolipids. The uridine diphosphate activated form of this sugar (UDP-GlcNAc) is the universal GlcNAc donor for the biosynthesis of all these structures, as well as the precursor for many modified acetamido sugars. Inhibitors of bacterial protein synthesis cause an increase in the concentrations of peptidoglycan precursors, including UDP-GlcNAc.
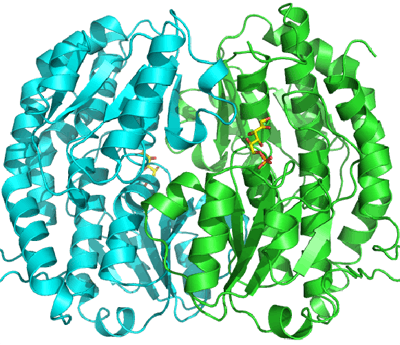
Structural studies
As of late 2007, 12 structures have been solved for this class of enzymes, with PDB accession codes 1JXA, 1MOQ, 1MOR, 1MOS, 1XFF, 1XFG, 2BPL, 2J6H, 2POC, 2PUT, 2PUV, and 2PUW.
References
- Ghosh S, Blumenthal HJ, Davidson E, Roseman S (1960). "Glucosamine metabolism. V. Enzymatic synthesis of glucosamine 6-phosphate". J. Biol. Chem. 235: 1265–1273. PMID 13827775.
- GRYDER RM, POGELL BM (1960). "Further studies on glucosamine 6-phosphate synthesis by rat liver enzymes". J. Biol. Chem. 235: 558–62. PMID 13829889.
- LELOIR LF, CARDINI CE (1953). "The biosynthesis of glucosamine". Biochim. Biophys. Acta. 12 (1-2): 15–22. doi:10.1016/0006-3002(53)90119-X. PMID 13115409.
- Teplyakov A, Obmolova G, Badet-Denisot MA, Badet B (1999). "The mechanism of sugar phosphate isomerization by glucosamine 6-phosphate synthase". Protein. Sci. 8 (3): 596–602. doi:10.1110/ps.8.3.596. PMC 2144271
 . PMID 10091662.
. PMID 10091662.