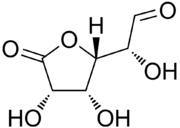
Glucuronolactone
 | |
-3D-balls.png) | |
| Names | |
|---|---|
| IUPAC name
(2R)-2-[(2S,3R,4S)-3,4-Dihydroxy-5-oxo-tetrahydrofuran-2-yl]-2-hydroxy-acetaldehyde | |
| Other names
Glucuronic acid lactone; Glucurone; Glucurolactone; D-Glucurono-3,6-lactone; D-glucurono-gamma-lactone | |
| Identifiers | |
| 32449-92-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 83317 |
| ECHA InfoCard | 100.046.397 |
| PubChem | 92283 |
| |
| |
| Properties | |
| C6H8O6 | |
| Molar mass | 176.12 g·mol−1 |
| Density | 1.76 g/cm3 (30 °C) |
| Melting point | 176 to 178 °C (349 to 352 °F; 449 to 451 K) |
| 26.9 g/100 mL | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Glucuronolactone is a naturally occurring chemical that is an important structural component of nearly all connective tissues.[1] Glucuronolactone is also found in many plant gums.[1]
Physical and chemical properties
Glucuronolactone is a white solid odorless compound, soluble in hot and cold water. Its melting point ranges from 176 to 178 °C.[1] The compound can exist in a monocyclic aldehyde form or in a bicyclic hemiacetal (lactol) form.
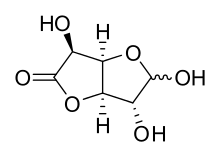 Lactol form of glucuronolactone
Lactol form of glucuronolactone
History
Glucuronolactone has received some notoriety due to an urban legend that it was a Vietnam War-era drug manufactured by the American government and since banned due to brain tumor-related deaths. However, the quoted British Medical Journal article does not exist; furthermore, no warnings appear on the Food and Drug Administration website regarding its potential to cause brain tumors or other maladies.[2] Moreover, glucuronolactone is hydrolyzed in the body (like butyrolactone) to glucuronic acid, which may be oxidized to glucaric acid, or isomerized to another hexuronic acid, so there is no reasonable toxicity mechanism.
Uses
Glucuronolactone is metabolized to glucaric acid, xylitol, and L-xylulose, and humans may also be able to use glucuronolactone as a precursor for ascorbic acid synthesis.[3] According to The Merck Index, it is also used as a detoxicant.[4] The liver uses glucose to create glucuronolactone, which inhibits the enzyme B-glucuronidase (metabolizes glucuronides), which should cause blood-glucuronide levels to rise. Glucuronides combine with toxic substances, such as morphine and depot medroxyprogesterone acetate, by converting them to water-soluble glucuronide-conjugates which are excreted in the urine.[5] Theoretically, higher blood-glucuronides should help remove toxins from the body, leading to the claim that energy drinks are detoxifying.[6] Free glucuronic acid (or its self-ester glucuronolactone) has less effect on detoxification than glucose , because the body synthesizes UDP-glucuronic acid from glucose. Therefore, sufficient carbohydrate intake provides enough UDP-glucuronic acid for detoxication , and foods rich in glucose are usually abundant in developed nations.
Glucuronolactone is a popular ingredient in energy drinks with claims that it detoxifies the body. Although levels of glucuronolactone in energy drinks can far exceed those found in the rest of the diet, the European Food Safety Authority (EFSA) has concluded that exposure to glucuronolactone from regular consumption of energy drinks is not a safety concern. The no-observed-adverse-effect level of glucuronolactone is 1000 mg/kg/day.[7]
See also
References
- 1 2 3 4 Merck Index, 11th Edition, 4362
- ↑ "Bull Marketed". Snopes.
- ↑ Baker EM, Bierman EL, Plough IC (1960). "Effect of D-glucuronic acid and D-glucuronolactone on ascorbic acid levels in blood and urine of man and dog". Am J Clin Nutr. 8: 369–73. PMID 13795987.
- ↑ Merck Index, 14th ed., 4467
- ↑ "Depo-Provera (Medroxyprogesterone) Drug Information: Clinical Pharmacology - Prescribing Information at RxList". RxList. Retrieved 25 August 2015.
- ↑ http://pilarmartinescudero.es/pdf/dopaje/Microgramredbull.pdf
- ↑ "EFSA - Opinion of the Scientific Committee/Scientific Panel: The use of taurine and D-glucurono-gamma-lactone as constituents of the so-called "energy" drinks". European Food Safety Authority. Retrieved 2012-05-04.