Glycine—tRNA ligase
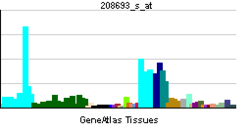
| View/Edit Human | View/Edit Mouse |
Glycine—tRNA ligase also known as glycyl-tRNA synthetase is an enzyme that in humans is encoded by the GARS gene.[3][4][5]
Function
This gene encodes glycyl-tRNA synthetase, one of the aminoacyl-tRNA synthetases that charge tRNAs with their cognate amino acids. The encoded enzyme is an (alpha)2 dimer which belongs to the class II family of tRNA synthetases.[5]
Reaction
| glycine-tRNA ligase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 6.1.1.14 | ||||||||
| CAS number | 9037-62-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
In enzymology, a glycine-tRNA ligase (EC 6.1.1.14) is an enzyme that catalyzes the chemical reaction
- ATP + glycine + tRNAGly AMP + diphosphate + glycyl-tRNAGly
The 3 substrates of this enzyme are ATP, glycine, and tRNA(Gly), whereas its 3 products are AMP, diphosphate, and glycyl-tRNA(Gly).
This enzyme belongs to the family of ligases, to be specific those forming carbon-oxygen bonds in aminoacyl-tRNA and related compounds. The systematic name of this enzyme class is glycine:tRNAGly ligase (AMP-forming). Other names in common use include glycyl-tRNA synthetase, glycyl-transfer ribonucleate synthetase, glycyl-transfer RNA synthetase, glycyl-transfer ribonucleic acid synthetase, and glycyl translase. This enzyme participates in glycine, serine and threonine metabolism and aminoacyl-trna biosynthesis.
Interactions
Glycyl-tRNA synthetase has been shown to interact with EEF1D.[6]
Clinical relevance
Glycyl-tRNA synthetase has been shown to be a target of autoantibodies in the human autoimmune diseases, polymyositis or dermatomyositis.[5]
The peripheral neuropathy Charcot-Marie-Tooth disease type 2D (CMT2D) has been liked to dominant mutations in GARS.[7] CMT2D usually manifests during the teenage years, and results in muscle weakness predominantly in the hands and feet.[8] Two mouse models of CMT2D have been used to better understand the disease, identifying that the disorder is caused by a toxic gain of function of the mutant glycine-tRNA ligase protein.[9] The CMT2D mice display peripheral nerve axon degeneration [10][11] and defective development of the neuromuscular junction.[12]
See also
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Nichols RC, Pai SI, Ge Q, Targoff IN, Plotz PH, Liu P (Nov 1995). "Localization of two human autoantigen genes by PCR screening and in situ hybridization--glycyl-tRNA synthetase locates to 7p15 and alanyl-tRNA synthetase locates to 16q22". Genomics. 30 (1): 131–2. doi:10.1006/geno.1995.0028. PMID 8595897.
- ↑ Ionasescu V, Searby C, Sheffield VC, Roklina T, Nishimura D, Ionasescu R (Sep 1996). "Autosomal dominant Charcot-Marie-Tooth axonal neuropathy mapped on chromosome 7p (CMT2D)". Human Molecular Genetics. 5 (9): 1373–5. doi:10.1093/hmg/5.9.1373. PMID 8872480.
- 1 2 3 "Entrez Gene: GARS glycyl-tRNA synthetase".
- ↑ Sang Lee J, Gyu Park S, Park H, Seol W, Lee S, Kim S (Feb 2002). "Interaction network of human aminoacyl-tRNA synthetases and subunits of elongation factor 1 complex". Biochemical and Biophysical Research Communications. 291 (1): 158–64. doi:10.1006/bbrc.2002.6398. PMID 11829477.
- ↑ Motley WW, Talbot K, Fischbeck KH (Feb 2010). "GARS axonopathy: not every neuron's cup of tRNA". Trends in Neurosciences. 33 (2): 59–66. doi:10.1016/j.tins.2009.11.001. PMC 2822721
 . PMID 20152552.
. PMID 20152552. - ↑ Sivakumar K, Kyriakides T, Puls I, Nicholson GA, Funalot B, Antonellis A, Sambuughin N, Christodoulou K, Beggs JL, Zamba-Papanicolaou E, Ionasescu V, Dalakas MC, Green ED, Fischbeck KH, Goldfarb LG (Oct 2005). "Phenotypic spectrum of disorders associated with glycyl-tRNA synthetase mutations". Brain. 128 (Pt 10): 2304–14. doi:10.1093/brain/awh590. PMID 16014653.
- ↑ Motley WW, Seburn KL, Nawaz MH, Miers KE, Cheng J, Antonellis A, Green ED, Talbot K, Yang XL, Fischbeck KH, Burgess RW (Dec 2011). "Charcot-Marie-Tooth-linked mutant GARS is toxic to peripheral neurons independent of wild-type GARS levels". PLoS Genetics. 7 (12): e1002399. doi:10.1371/journal.pgen.1002399. PMC 3228828
 . PMID 22144914.
. PMID 22144914. - ↑ Seburn KL, Nangle LA, Cox GA, Schimmel P, Burgess RW (Sep 2006). "An active dominant mutation of glycyl-tRNA synthetase causes neuropathy in a Charcot-Marie-Tooth 2D mouse model". Neuron. 51 (6): 715–26. doi:10.1016/j.neuron.2006.08.027. PMID 16982418.
- ↑ Achilli F, Bros-Facer V, Williams HP, Banks GT, AlQatari M, Chia R, Tucci V, Groves M, Nickols CD, Seburn KL, Kendall R, Cader MZ, Talbot K, van Minnen J, Burgess RW, Brandner S, Martin JE, Koltzenburg M, Greensmith L, Nolan PM, Fisher EM (Jul–Aug 2009). "An ENU-induced mutation in mouse glycyl-tRNA synthetase (GARS) causes peripheral sensory and motor phenotypes creating a model of Charcot-Marie-Tooth type 2D peripheral neuropathy". Disease Models & Mechanisms. 2 (7-8): 359–73. doi:10.1242/dmm.002527. PMC 2707104
 . PMID 19470612.
. PMID 19470612. - ↑ Sleigh JN, Grice SJ, Burgess RW, Talbot K, Cader MZ (May 2014). "Neuromuscular junction maturation defects precede impaired lower motor neuron connectivity in Charcot-Marie-Tooth type 2D mice". Human Molecular Genetics. 23 (10): 2639–50. doi:10.1093/hmg/ddt659. PMID 24368416.
Further reading
- Fraser MJ (May 1963). "Glycyl-RNA synthetase of rat liver: partial purification and effects of some metal ions on its activity". Canadian Journal of Biochemistry and Physiology. 41: 1123–33. doi:10.1139/o63-128. PMID 13959340.
- Niyomporn B, Dahl JL, Strominger JL (Feb 1968). "Biosynthesis of the peptidoglycan of bacterial cell walls. IX. Purification and properties of glycyl transfer ribonucleic acid synthetase from Staphylococcus aureus". The Journal of Biological Chemistry. 243 (4): 773–8. PMID 4295604.
- Hipps D, Shiba K, Henderson B, Schimmel P (Jun 1995). "Operational RNA code for amino acids: species-specific aminoacylation of minihelices switched by a single nucleotide". Proceedings of the National Academy of Sciences of the United States of America. 92 (12): 5550–2. doi:10.1073/pnas.92.12.5550. PMC 41733
 . PMID 7539919.
. PMID 7539919. - Williams J, Osvath S, Khong TF, Pearse M, Power D (Apr 1995). "Cloning, sequencing and bacterial expression of human glycine tRNA synthetase". Nucleic Acids Research. 23 (8): 1307–10. doi:10.1093/nar/23.8.1307. PMC 306854
 . PMID 7753621.
. PMID 7753621. - Ge Q, Trieu EP, Targoff IN (Nov 1994). "Primary structure and functional expression of human Glycyl-tRNA synthetase, an autoantigen in myositis". The Journal of Biological Chemistry. 269 (46): 28790–7. PMID 7961834.
- Shiba K, Schimmel P, Motegi H, Noda T (Nov 1994). "Human glycyl-tRNA synthetase. Wide divergence of primary structure from bacterial counterpart and species-specific aminoacylation". The Journal of Biological Chemistry. 269 (47): 30049–55. PMID 7962006.
- Rho SB, Lee KH, Kim JW, Shiba K, Jo YJ, Kim S (Sep 1996). "Interaction between human tRNA synthetases involves repeated sequence elements". Proceedings of the National Academy of Sciences of the United States of America. 93 (19): 10128–33. doi:10.1073/pnas.93.19.10128. PMC 38348
 . PMID 8816763.
. PMID 8816763. - Mudge SJ, Williams JH, Eyre HJ, Sutherland GR, Cowan PJ, Power DA (Mar 1998). "Complex organisation of the 5'-end of the human glycine tRNA synthetase gene". Gene. 209 (1-2): 45–50. doi:10.1016/S0378-1119(98)00007-9. PMID 9524218.
- Kneussel M, Hermann A, Kirsch J, Betz H (Mar 1999). "Hydrophobic interactions mediate binding of the glycine receptor beta-subunit to gephyrin". Journal of Neurochemistry. 72 (3): 1323–6. doi:10.1046/j.1471-4159.1999.0721323.x. PMID 10037506.
- Sang Lee J, Gyu Park S, Park H, Seol W, Lee S, Kim S (Feb 2002). "Interaction network of human aminoacyl-tRNA synthetases and subunits of elongation factor 1 complex". Biochemical and Biophysical Research Communications. 291 (1): 158–64. doi:10.1006/bbrc.2002.6398. PMID 11829477.
- Antonellis A, Ellsworth RE, Sambuughin N, Puls I, Abel A, Lee-Lin SQ, Jordanova A, Kremensky I, Christodoulou K, Middleton LT, Sivakumar K, Ionasescu V, Funalot B, Vance JM, Goldfarb LG, Fischbeck KH, Green ED (May 2003). "Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V". American Journal of Human Genetics. 72 (5): 1293–9. doi:10.1086/375039. PMC 1180282
 . PMID 12690580.
. PMID 12690580. - Del Bo R, Locatelli F, Corti S, Scarlato M, Ghezzi S, Prelle A, Fagiolari G, Moggio M, Carpo M, Bresolin N, Comi GP (Mar 2006). "Coexistence of CMT-2D and distal SMA-V phenotypes in an Italian family with a GARS gene mutation". Neurology. 66 (5): 752–4. doi:10.1212/01.wnl.0000201275.18875.ac. PMID 16534118.
- James PA, Cader MZ, Muntoni F, Childs AM, Crow YJ, Talbot K (Nov 2006). "Severe childhood SMA and axonal CMT due to anticodon binding domain mutations in the GARS gene". Neurology. 67 (9): 1710–2. doi:10.1212/01.wnl.0000242619.52335.bc. PMID 17101916.
- Xie W, Schimmel P, Yang XL (Dec 2006). "Crystallization and preliminary X-ray analysis of a native human tRNA synthetase whose allelic variants are associated with Charcot-Marie-Tooth disease". Acta Crystallographica Section F. 62 (Pt 12): 1243–6. doi:10.1107/S1744309106046434. PMC 2225372
 . PMID 17142907.
. PMID 17142907. - Cader MZ, Ren J, James PA, Bird LE, Talbot K, Stammers DK (Jun 2007). "Crystal structure of human wildtype and S581L-mutant glycyl-tRNA synthetase, an enzyme underlying distal spinal muscular atrophy". FEBS Letters. 581 (16): 2959–64. doi:10.1016/j.febslet.2007.05.046. PMID 17544401.
- Xie W, Nangle LA, Zhang W, Schimmel P, Yang XL (Jun 2007). "Long-range structural effects of a Charcot-Marie-Tooth disease-causing mutation in human glycyl-tRNA synthetase". Proceedings of the National Academy of Sciences of the United States of America. 104 (24): 9976–81. doi:10.1073/pnas.0703908104. PMC 1891255
 . PMID 17545306.
. PMID 17545306.
External links
- GeneReviews/NCBI/NIH/UW entry on Charcot-Marie-Tooth Neuropathy Type 2
- GeneReviews/NCBI/NIH/UW entry on GARS-Associated Axonal Neuropathy, Charcot-Marie-Tooth Neuropathy Type 2D, Distal Spinal Muscular Atrophy V