Folding funnel
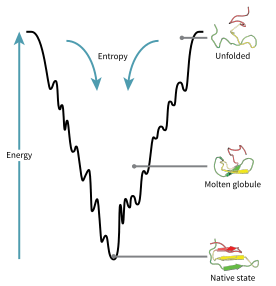
The folding funnel hypothesis is a specific version of the energy landscape theory of protein folding, which assumes that a protein's native state corresponds to its free energy minimum under the solution conditions usually encountered in cells. Although energy landscapes may be "rough", with many non-native local minima in which partially folded proteins can become trapped, the folding funnel hypothesis assumes that the native state is a deep free energy minimum with steep walls, corresponding to a single well-defined tertiary structure.
The folding funnel hypothesis is closely related to the hydrophobic collapse hypothesis, under which the driving force for protein folding is the stabilization associated with the sequestration of hydrophobic amino acid side chains in the interior of the folded protein. This allows the water solvent to maximize its entropy, lowering the total free energy. On the side of the protein, free energy is further lowered by favorable energetic contacts: isolation of electrostatically charged side chains on the solvent-accessible protein surface and neutralization of salt bridges within the protein's core. The molten globule state predicted by the folding funnel theory as an ensemble of folding intermediates thus corresponds to a protein in which hydrophobic collapse has occurred but many native contacts, or close residue-residue interactions represented in the native state, have yet to form.
In the canonical depiction of the folding funnel, the depth of the well represents the energetic stabilization of the native state versus the denatured state, and the width of the well represents the conformational entropy of the system. The surface outside the well is shown as relatively flat to represent the heterogeneity of the random coil state. The theory's name derives from an analogy between the shape of the well and a physical funnel, in which dispersed liquid is concentrated into a single narrow area.
See also
- Protein structure prediction
- Levinthal paradox
- Chaperone — proteins that assist other proteins with folding or unfolding
References
- Dobson CM (2000-12-15). "The nature and significance of protein folding". In RH Pain. Mechanisms of Protein Folding (2nd ed.). Oxford, UK: Oxford University Press. ISBN 0-19-963788-1.
- Matagne A, Chung E, Ball LJ, Radford SE, Robinson CV, Dobson CM (1998). "The origin of the alpha-domain intermediate in the folding of hen lysozyme". J Mol Biol. 277 (5): 997–1005. doi:10.1006/jmbi.1998.1657. PMID 9571017.