Fahr's syndrome
| Fahr's syndrome | |
|---|---|
 | |
| Idiopathic basal ganglia calcification | |
| Classification and external resources | |
| Specialty | neurology |
| ICD-10 | G23.8 |
| OMIM | 213600 |
| DiseasesDB | 32200 |
| GeneReviews | |
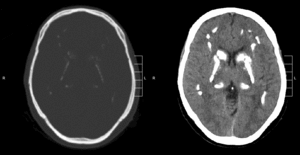
Idiopathic basal ganglia calcification, also known as Fahr disease, is a rare,[1] genetically dominant, inherited neurological disorder characterized by abnormal deposits of calcium in areas of the brain that control movement. Through the use of CT scans, calcifications are seen primarily in the basal ganglia and in other areas such as the cerebral cortex.[2]
Signs and symptoms
Symptoms of this disease include deterioration of motor functions and speech, seizures, and other involuntary movement. Other symptoms are headaches, dementia, and vision impairment. Characteristics of Parkinson's Disease are also similar to Fahr's Syndrome.[3]
The disease usually manifests itself in the third to fifth decade of life but may appear in childhood or later in life.[4] It usually presents with clumsiness, fatigability, unsteady gait, slow or slurred speech, difficulty swallowing, involuntary movements or muscle cramping. Seizures of various types are common. Neuropsychiatric symptoms, which may be the first or the most prominent manifestations, range from mild difficulty with concentration and memory to changes in personality and/or behavior, to psychosis and dementia.[5]
Causes
A locus at 14q has been suggested, but no gene has been identified.[6] A second locus has been identified on chromosome 8[7] and a third has been reported on chromosome 2.[8] This suggests there may be some genetic heterogeneity in this disease.[9]
A mutation in the gene encoding the type III sodium dependent phosphate transporter 2 (SLC20A2) located on chromosome 8 has been reported.[10] Biochemical evidence suggests that phosphate transport may be involved in this disease.
Two other genes have been associated with this condition: PDGFB and PDGFRB.[11] These genes are biochemically linked: PDGFBR encodes the platelet-derived growth factor receptor β and PDGFB encodes the ligand of PDGF-Rβ. These genes are active during angiogenesis to recruit pericytes which suggests that alterations in the blood brain barrier may be involved in the pathogenesis of this condition.
Basal ganglia calcification may occur as a consequence of several other known genetic conditions and these have to be excluded before a diagnosis can be made.[12][13][14][15]
Pathology
The most commonly affected region of the brain is the lenticular nucleus and in particular the internal globus pallidus.[16] Calcifications in the caudate, dentate nuclei, putamen and thalami are also common. Occasionally calcifications begin or predominate in regions outside the basal ganglia.
Calcification seems to be progressive, since calcifications are generally more extensive in older individuals and an increase in calcification can sometimes be documented on follow up of affected subjects.
As well as the usual sites the cerebellar gyri, brain stem, centrum semiovale and subcortical white matter may also be affected.
Diffuse atrophic changes with dilatation of the subarachnoid space and/or ventricular system may coexist with the calcifications.
Histologically concentric calcium deposits within the walls of small and medium-sized arteries are present. Less frequently the veins may also be affected. Droplet calcifications can be observed along capillaries. These deposits may eventually lead to closure of the lumina of vessels.
The pallidal deposits stain positively for iron. Diffuse gliosis may surround the large deposits but significant loss of nerve cells is rare.
On electron microscopy the mineral deposits appear as amorphous or crystalline material surrounded by a basal membrane. Calcium granules are seen within the cytoplasm of neuronal and glial cells.
The calcifications seen in this condition are indistinguishable from those secondary to hypoparathyroidism or other causes.
Diagnosis
In addition to the usual routine haematologic and biochemical investigations, the serum calcium, phosphorus, magnesium, alkaline phosphatase, calcitonin and parathyroid hormone should also be measured. The cerebrospinal fluid (CSF) should be examined to exclude bacteria, viruses and parasites.[17] The Ellsworth Howard test (a 10-20 fold increase of urinary cyclic AMP excretion following stimulation with 200 micromoles of parathyroid hormone) may be worth doing also. Serology for toxoplasmosis is also indicated.
Brain CT scan is the preferred method of localizing and assessing the extent of cerebral calcifications.
Elevated levels of copper, iron, magnesium and zinc but not calcium have been reported in the CSF but the significance of this finding — if any — is not known.[18]
The diagnosis requires the following criteria be met:
- the presence of bilateral calcification of the basal ganglia
- the presence of progressive neurologic dysfunction
- the absence of an alternative metabolic, infectious, toxic or traumatic cause
- a family history consistent with autosomal dominant inheritance
The calcification is usually identified on CT scan but may be visible on plain films of the skull.
Management
There is currently no cure for Fahr's Syndrome nor a standard course of treatment. The available treatment is directed symptomatic control. If parkinsonian features develop, there is generally poor response to levodopa therapy. Case reports have suggested that haloperidol or lithium carbonate may help with psychotic symptoms.[19] One case report described an improvement with the use of a bisphosphonate.[20]
Genetic counseling may be helpful.
Prognosis
The prognosis for any individual with Fahr's Syndrome is variable and hard to predict. There is no reliable correlation between age, extent of calcium deposits in the brain, and neurological deficit. Since the appearance of calcification is age-dependent, a CT scan could be negative in a gene carrier who is younger than the age of 55.[21]
Progressive neurological deterioration generally results in disability and death.
History
The disease was first noted by German neurologist Karl Theodor Fahr in 1930.[22][23] A less common name for the condition is Chavany-Brunhes syndrome and Fritsche's syndrome, the former named after Jacques Brunhes, Jean Alfred Émile Chavany, while the later named after R. Fritsche.[24][25]
Less than 20 families had been reported in the literature up to 1997.[26]
See also
References
- ↑ http://rarediseases.info.nih.gov/GARD/Disease.aspx?PageID=4&diseaseID=8272
- ↑ Benke T, Karner E, Seppi K, Delazer M, Marksteiner J, Donnemiller E (August 2004). "Subacute dementia and imaging correlates in a case of Fahr's disease". J. Neurol. Neurosurg. Psychiatr. 75 (8): 1163–5. doi:10.1136/jnnp.2003.019547. PMC 1739167
 . PMID 15258221.
. PMID 15258221. - ↑ "NINDS Fahr's Syndrome Information Page". National Institute of Neurological Disorders and Stroke. Retrieved 13 January 2007.
- ↑ Sobrido MJ, Hopfer S, Geschwind DH (2007) "Familial idiopathic basal ganglia calcification." In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. SourceGeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2004
- ↑ Chiu HF, Lam LC, Shum PP, Li KW (January 1993). "Idiopathic calcification of the basal ganglia". Postgrad Med J. 69 (807): 68–70. doi:10.1136/pgmj.69.807.68. PMC 2399589
 . PMID 8446558.
. PMID 8446558. - ↑ Geschwind DH, Loginov M, Stern JM (September 1999). "Identification of a locus on chromosome 14q for idiopathic basal ganglia calcification (Fahr disease)". Am. J. Hum. Genet. 65 (3): 764–72. doi:10.1086/302558. PMC 1377984
 . PMID 10441584.
. PMID 10441584. - ↑ Dai X, Gao Y, Xu Z, et al. (October 2010). "Identification of a novel genetic locus on chromosome 8p21.1-q11.23 for idiopathic basal ganglia calcification". Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B (7): 1305–10. doi:10.1002/ajmg.b.31102. PMID 20552677.
- ↑ Volpato CB, De Grandi A, Buffone E, et al. (November 2009). "2q37 as a susceptibility locus for idiopathic basal ganglia calcification (IBGC) in a large South Tyrolean family". J. Mol. Neurosci. 39 (3): 346–53. doi:10.1007/s12031-009-9287-3. PMID 19757205.
- ↑ Oliveira JR, Spiteri E, Sobrido MJ, et al. (December 2004). "Genetic heterogeneity in familial idiopathic basal ganglia calcification (Fahr disease)". Neurology. 63 (11): 2165–7. doi:10.1212/01.wnl.0000145601.88274.88. PMID 15596772.
- ↑ Wang C, Li Y, Shi L, et al. (March 2012). "Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis". Nat. Genet. 44 (3): 254–6. doi:10.1038/ng.1077. PMID 22327515.
- ↑ Westenberger A1, Klein C (2014) The genetics of primary familial brain calcifications. Curr Neurol Neurosci Rep 14(10):490 doi: 10.1007/s11910-014-0490-4
- ↑ Niwa A, Naito Y, Kuzuhara S (2008). "Severe cerebral calcification in a case of LEOPARD syndrome". Intern. Med. 47 (21): 1925–9. doi:10.2169/internalmedicine.47.1365. PMID 18981639.
- ↑ Preusser M, Kitzwoegerer M, Budka H, Brugger S (October 2007). "Bilateral striopallidodentate calcification (Fahr's syndrome) and multiple system atrophy in a patient with longstanding hypoparathyroidism". Neuropathology. 27 (5): 453–6. doi:10.1111/j.1440-1789.2007.00790.x. PMID 18018479.
- ↑ Saito Y, Shibuya M, Hayashi M, et al. (July 2005). "Cerebellopontine calcification: a new entity of idiopathic intracranial calcification?". Acta Neuropathol. 110 (1): 77–83. doi:10.1007/s00401-005-1011-y. PMID 15959794.
- ↑ Tojyo K, Hattori T, Sekijima Y, Yoshida K, Ikeda S (June 2001). "[A case of idiopathic brain calcification associated with dyschromatosis symmetrica hereditaria, aplasia of dental root, and aortic valve sclerosis]". Rinsho Shinkeigaku (in Japanese). 41 (6): 299–305. PMID 11771159.
- ↑ Bonazza S, La Morgia C, Martinelli P, Capellari S (August 2011). "Strio-pallido-dentate calcinosis: a diagnostic approach in adult patients". Neurol. Sci. 32 (4): 537–45. doi:10.1007/s10072-011-0514-7. PMID 21479613.
- ↑ Morita M, Tsuge I, Matsuoka H, et al. (May 1998). "Calcification in the basal ganglia with chronic active Epstein-Barr virus infection". Neurology. 50 (5): 1485–8. doi:10.1212/wnl.50.5.1485. PMID 9596016.
- ↑ Hozumi I, Kohmura A, Kimura A, et al. (2010). "High Levels of Copper, Zinc, Iron and Magnesium, but not Calcium, in the Cerebrospinal Fluid of Patients with Fahr's Disease". Case Rep Neurol. 2 (2): 46–51. doi:10.1159/000313920. PMC 2905580
 . PMID 20671856.
. PMID 20671856. - ↑ Munir KM (February 1986). "The treatment of psychotic symptoms in Fahr's disease with lithium carbonate". J Clin Psychopharmacol. 6 (1): 36–8. doi:10.1097/00004714-198602000-00008. PMID 3081601.
- ↑ Loeb JA (March 1998). "Functional improvement in a patient with cerebral calcinosis using a bisphosphonate". Mov. Disord. 13 (2): 345–9. doi:10.1002/mds.870130225. PMID 9539353.
- ↑ "NINDS Fahr's Syndrome Information Page". National Institute of Neurological Disorders and Stroke. Retrieved 13 February 2007.
- ↑ Fahr, T. (1930–1931). "Idiopathische Verkalkung der Hirngefässe". Zentralblatt für allgemeine Pathologie und pathologische Anatomie. 50: 129–133.
- ↑ Fahr's disease at Who Named It?
- ↑ Chavany-Brunhes syndrome at Who Named It?
- ↑ http://rarediseases.info.nih.gov/GARD/QnA.aspx?PageID=4&CaseID=22373&DiseaseID=8272
- ↑ Kobari M, Nogawa S, Sugimoto Y, Fukuuchi Y (March 1997). "Familial idiopathic brain calcification with autosomal dominant inheritance". Neurology. 48 (3): 645–9. doi:10.1212/wnl.48.3.645. PMID 9065541.
External links
- Fahr Syndrome Images (MedPix)
- National Organization for Rare Disorders (NORD)
- National Institute on Aging (NIA)
- National Institute of Mental Health (NIMH)
- Fahr Syndrome Images (iNeurologist)