Favorskii rearrangement
The Favorskii rearrangement, named for the Russian chemist Alexei Yevgrafovich Favorskii, is most principally a rearrangement of cyclopropanones and α-halo ketones which leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α’-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.[1][2][3][4][5][6][7][8]
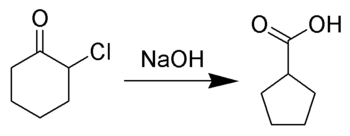
Reaction mechanism
The reaction mechanism is thought to involve the formation of an enolate on the side of the ketone away from the chlorine atom. This enolate cyclizes to a cyclopropanone intermediate which is then attacked by the hydroxide nucleophile.
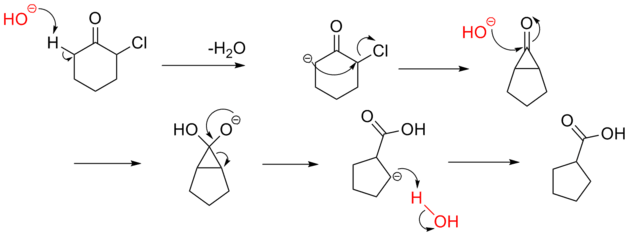
Usage of alkoxide anions such as sodium methoxide, instead of sodium hydroxide, yields the ring-contracted ester product.
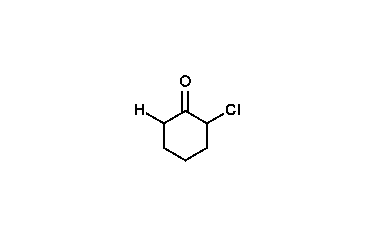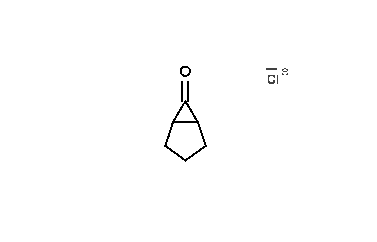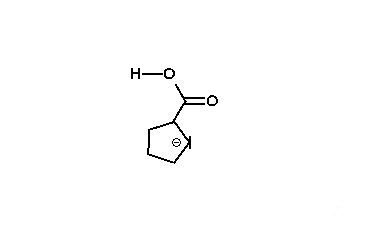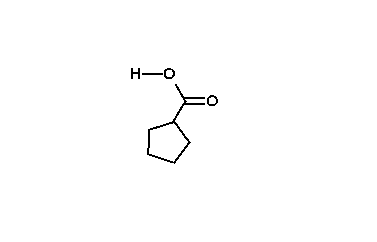 |
| An animation of the reaction mechanism |
Wallach degradation
In the related Wallach degradation (Otto Wallach, 1918) not one but two halogen atoms flank the ketone resulting in a new contracted ketone after oxidation and decarboxylation[9][10]
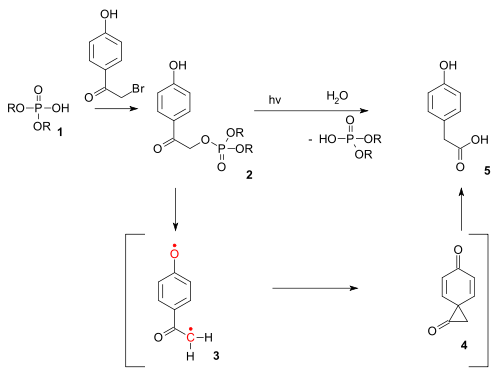
Photo-Favorskii reaction
The reaction type also exists as a photochemical reaction. The photo-Favorskii reaction has been used in the photochemical unlocking of certain phosphates (for instance those of ATP) protected by so-called p-hydroxyphenacyl groups.[11] The deprotection proceeds through a triplet diradical (3) and a dione spiro intermediate (4) although the latter has thus far eluded detection.[12]
See also
- A classic cubane synthesis contains two Favorskii rearrangements.
- Trimethylenemethane cycloaddition which can proceed via a similar mechanism
References
- ↑ Favorskii, A. E. J. Russ. Phys. Chem. Soc. 1894, 26, 590.
- ↑ Favorskii, A. E. J. Russ. Phys. Chem. Soc. 1905, 37, 643.
- ↑ Favorskii, A. E. J. Prakt. Chem. 1913, 88, 658.
- ↑ Cope, Arthur (1960). Organic Reaction Volume XI (1 ed.). Newyork: Wiley-Interscience. doi:10.1002/jps.2600500225. ISBN 9780471171270.
- ↑ Wohllebe, J.; Garbisch, E. W. (1988). "Ring Contraction via A Favorskii-Type Rearrangement: Cycloudecanone". Organic Syntheses. 6: 368. doi:10.15227/orgsyn.056.0107.
- ↑ Shioiri, Takayuki; Kawai, Nobutaka (1978). "New methods and reagents in organic synthesis. 2. A facile conversion of alkyl aryl ketones to .alpha.-arylalkanoic acids using diphenyl phosphorazidate. Its application to a new synthesis of ibuprofen and naproxen, nonsteroidal antiinflammatory agents". The Journal of Organic Chemistry. 43 (14): 2936–2938. doi:10.1021/jo00408a049.
- ↑ Hamada, Yasumasa; Shioiri, Takayuki (1990). "Cycloundecanecarboxylic Acid". Organic Syntheses. 7: 135. doi:10.15227/orgsyn.062.0191.
- ↑ Goheen, D. W.; Vaughan, W. R. (1693). "Cyclopentanecarboxylic acid, methyl ester". Organic Syntheses. 4: 594. doi:10.15227/orgsyn.039.0037.
- ↑ Zur Kenntnis der Terpene und der ätherischen Öle. Über das Verhalten zweifach gebromter hexacyclischer Ketone in Abhängigkeit von der Stellung der Bromatome Justus Liebig's Annalen der Chemie Volume 414, Issue 3 , Pages 271 – 296 O. Wallach 1918 doi:10.1002/jlac.19184140303
- ↑ Zur Kenntnis der Terpene und der ätherischen Öle (pp. 296–366) O. Wallach Justus Liebig's Annalen der ChemieVolume 414, Issue 3 , pp. 296–366 1918 doi:10.1002/jlac.19184140304
- ↑ New Photoactivated Protecting Groups. 6. p-Hydroxyphenacyl: A Phototrigger for Chemical and Biochemical Probes Chan-Ho Park and Richard S. Givens J. Am. Chem. Soc.; 1997; 119(10) pp 2453–63; (Article) doi:10.1021/ja9635589
- ↑ The Photo-Favorskii Reaction of p-Hydroxyphenacyl Compounds Is Initiated by Water-Assisted, Adiabatic Extrusion of a Triplet Biradical Richard S. Givens, Dominik Heger, Bruno Hellrung, Yavor Kamdzhilov, Marek Mac, Peter G. Conrad, II, Elizabeth Cope, Jong I. Lee, Julio F. Mata-Segreda, Richard L. Schowen and Jakob Wirz J. AM. CHEM. SOC. 2008, 130, 3307–09 doi:10.1021/ja7109579