Inositol oxygenase
| myo-inositol oxygenase | |
|---|---|
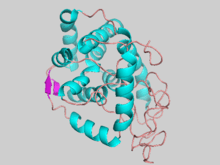 Structure of the mouse myo-inositol oxygenase monomer, generated from 2HUO, colored by secondary structure element. | |
| Identifiers | |
| Symbol | MIOX |
| Alt. symbols | ALDRL6 |
| Entrez | 55586 |
| HUGO | 14522 |
| OMIM | 606774 |
| PDB | 2IBN |
| RefSeq | NM_017584 |
| UniProt | Q9UGB7 |
| Other data | |
| EC number | 1.13.99.1 |
| Locus | Chr. 22 q |
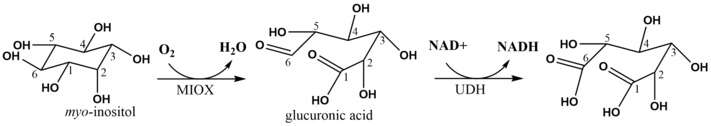
Inositol oxygenase, also commonly referred to as myo-inositol oxygenase (MIOX), is a non-heme di-iron enzyme that oxidizes myo-inositol to glucuronic acid.[1] The enzyme employs a unique four-electron transfer at its Fe(II)/Fe(III) coordination sites and the reaction proceeds through the direct binding of myo-inositol followed by attack of the iron center by diatomic oxygen. This enzyme is part of the only known pathway for the catabolism of inositol in humans[2] and is expressed primarily in the kidneys.[3][4] Recent medical research regarding MIOX has focused on understanding its role in metabolic and kidney diseases such as diabetes, obesity and acute kidney injury. Industrially-focused engineering efforts are centered on improving MIOX activity in order to produce glucaric acid in heterologous hosts.
Structure
Myo-inositol oxygenase is a monomeric 33 kDa protein in both solution and crystal.[5] This enzyme possesses a Fe(II)/Fe(III) atomic pair at the catalytic active site which enables its unique four-electron transfer mechanism. Recent crystallization studies have elucidated the structures of the mouse MIOX [5] in 2006 followed by the human MIOX[6] in 2008.
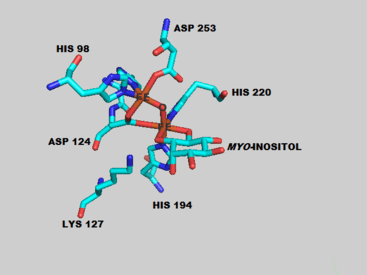
The overall structure of the mouse MIOX is primarily helical with five alpha helices forming the core of the protein.[5] Like other di-iron oxygenases, the iron coordination centers are buried deep inside the protein presumably to protect the cell from the superoxide and radical reaction intermediates that are formed.[7] The two iron centers are coordinated by various amino acids and water molecules as shown in complex with the myo-inositol substrate. The human MIOX structure superimposes closely onto the mouse MIOX structure, sharing 86% sequence identity over the structural alignment but with some differences in the residues surrounding the active site.[6] The human enzyme is characterized by eight alpha helices and a small anti-parallel two-stranded beta sheet.[6]
the MIOX protein fold diverges from that of other non-heme di-iron oxygenases including ribonucleotide reductase and soluble methane monooxygenase.[8] Instead, MIOX closely resembles proteins in the HD-domain superfamily based on its highly conserved metal binding strategy and the presence of the four His ligands on the iron center.[5]
Mechanism
MIOX can accept D-myo-inositol as well as the less abundant chiro isomer of inositol as substrates.[9] A series of crystallization, spectroscopy and density functional theory experiments have revealed a putative mechanism (shown right) for the oxidation of myo-inositol.[10][11][12] ENDOR spectroscopy was used to determine that the substrate directly binds to the Fe(II)/Fe(III) di-iron center of MIOX most likely through the O1 atom of myo-inositol.[7] In the mouse MIOX, this binding process was shown to be dependent on proximal amino acid residues as alanine mutants D85A and K127A were unable to turnover substrate.[5] This binding step positions the myo-inositol prior to the catalytic steps which involve attack of an iron center by diatomic oxygen followed by abstraction of a myo-inositol hydrogen atom.
A superoxide Fe(III)/Fe(III) species is formed as diatomic oxygen displaces water as a coordinating ligand on one of the Fe atoms. Next, the hydrogen atom from C1 of myo-inositol is abstracted to generate a radical that can be attacked by an oxygen radical. Release of D-glucuronic acid is achieved in the fourth step.
Biological Function
Myo-inositol can be ingested from fruits and vegetables and actively transported into cells or instead directly synthesized from glucose.[13] In the kidney, MIOX converts myo-inositol to glucuronic acid which is then able to enter the glucuronate-xylulose pathway for conversion to xylulose-5-phosphate.[13] This product can then easily enter the pentose phosphate pathway. Hence, MIOX enables the conversion and catabolism of inositol to generate NADPH and other pentose sugars.
Disease Relevance.
Myo-inositol is a component of the inositol phosphates and phosphoinositides that serve as secondary messengers in many cellular processes including insulin action. Due to its exclusive expression in the kidney, research has focused on understanding the potential role of both myo-inositol levels and MIOX activity on metabolic diseases like diabetes mellitus and obesity. Depletion of MIOX and accumulation of polyols, such as inositol and xylitol, have been cited as contributing factors in complications associated with diabetes.[14] Additionally, a recent study has shown that MIOX is upregulated in the diabetic state with its transcription heavily regulated by osmolarity, glucose levels and oxidative stress.[15] This upregulation is associated with the formation of reactive oxidative species that lead to interstitial injury in the kidney.[15]
There is also interest in evaluating MIOX expression as a potential biomarker of acute kidney injury. MIOX expression was shown to increase in the serum of animals and plasma of critically ill patients within 24 hours of acute kidney injury specifically.[16] An immunoassay of MIOX expression may potentially predict these life-threatening injuries earlier than the current diagnostic—detection of plasma creatine.
Industrial Relevance
The MIOX enzyme has been the object of intense metabolic engineering efforts to produce glucaric acid through biosynthetic pathways. In 2004, the U.S. Department of Energy released a list of the top value-added chemicals from biomass which included glucaric acid—the direct product of the oxidation of glucuronic acid. The first biosynthetic production of glucaric acid was achieved in 2009 with use of the uronate dehydrogenase (UDH) enzyme.[17] Since then, the MIOX enzyme has been engineered for improved glucaric acid production through numerous strategies including appendage of an N-terminal SUMO-tag, directed evolution[18] and also the use of modular, synthetic scaffolds to increase its effective local concentration.
See also
References
- ↑ Bollinger JM, Diao Y, Matthews ML, Xing G, Krebs C (Feb 2009). "myo-Inositol oxygenase: a radical new pathway for O(2) and C-H activation at a nonheme diiron cluster". Dalton Transactions (6): 905–14. doi:10.1039/b811885j. PMID 19173070.
- ↑ Hankes LV, Politzer WM, Touster O, Anderson L (Oct 1969). "Myo-inositol catabolism in human pentosurics: the predominant role of the glucuronate-xylulose-pentose phosphate pathway". Annals of the New York Academy of Sciences. 165 (2): 564–76. doi:10.1111/j.1749-6632.1970.tb56424.x. PMID 5259614.
- ↑ Reddy CC, Swan JS, Hamilton GA (Aug 1981). "myo-Inositol oxygenase from hog kidney. I. Purification and characterization of the oxygenase and of an enzyme complex containing the oxygenase and D-glucuronate reductase". The Journal of Biological Chemistry. 256 (16): 8510–8. PMID 7263666.
- ↑ Charalampous FC (Feb 1959). "Biochemical studies on inositol. V. Purification and properties of the enzyme that cleaves inositol to D-glucuronic acid". The Journal of Biological Chemistry. 234 (2): 220–7. PMID 13630882.
- 1 2 3 4 5 Brown, Peter M.; Caradoc-Davies, Tom T.; Dickson, James M. J.; Cooper, Garth J. S.; Loomes, Kerry M.; Baker, Edward N. (2006-10-10). "Crystal structure of a substrate complex of myo-inositol oxygenase, a di-iron oxygenase with a key role in inositol metabolism". Proceedings of the National Academy of Sciences of the United States of America. 103 (41): 15032–15037. doi:10.1073/pnas.0605143103. ISSN 0027-8424. PMC 1622774
 . PMID 17012379.
. PMID 17012379. - 1 2 3 Thorsell, Ann-Gerd; Persson, Camilla; Voevodskaya, Nina; Busam, Robert D.; Hammarström, Martin; Gräslund, Susanne; Gräslund, Astrid; Hallberg, B. Martin (2008-05-30). "Structural and biophysical characterization of human myo-inositol oxygenase". The Journal of Biological Chemistry. 283 (22): 15209–15216. doi:10.1074/jbc.M800348200. ISSN 0021-9258. PMC 3258897
 . PMID 18364358.
. PMID 18364358. - 1 2 Kim, Sun Hee; Xing, Gang; Bollinger, J. Martin; Krebs, Carsten; Hoffman, Brian M. (2006-08-16). "Demonstration by 2H ENDOR spectroscopy that myo-inositol binds via an alkoxide bridge to the mixed-valent diiron center of myo-inositol oxygenase". Journal of the American Chemical Society. 128 (32): 10374–10375. doi:10.1021/ja063602c. ISSN 0002-7863. PMID 16895396.
- ↑ Hirao, Hajime; Morokuma, Keiji (2009-12-02). "Insights into the (superoxo)Fe(III)Fe(III) intermediate and reaction mechanism of myo-inositol oxygenase: DFT and ONIOM(DFT:MM) study". Journal of the American Chemical Society. 131 (47): 17206–17214. doi:10.1021/ja905296w. ISSN 1520-5126. PMID 19929019.
- ↑ Arner RJ, Prabhu KS, Thompson JT, Hildenbrandt GR, Liken AD, Reddy CC (Dec 2001). "myo-Inositol oxygenase: molecular cloning and expression of a unique enzyme that oxidizes myo-inositol and D-chiro-inositol". The Biochemical Journal. 360 (Pt 2): 313–20. doi:10.1042/0264-6021:3600313. PMC 1222231
 . PMID 11716759.
. PMID 11716759. - ↑ Xing, Gang; Barr, Eric W.; Diao, Yinghui; Hoffart, Lee M.; Prabhu, K. Sandeep; Arner, Ryan J.; Reddy, C. Channa; Krebs, Carsten; Bollinger, J. Martin (2006-05-02). "Oxygen activation by a mixed-valent, diiron(II/III) cluster in the glycol cleavage reaction catalyzed by myo-inositol oxygenase". Biochemistry. 45 (17): 5402–5412. doi:10.1021/bi0526276. ISSN 0006-2960. PMID 16634621.
- ↑ Xing, Gang; Hoffart, Lee M.; Diao, Yinghui; Prabhu, K. Sandeep; Arner, Ryan J.; Reddy, C. Channa; Krebs, Carsten; Bollinger, J. Martin (2006-05-02). "A coupled dinuclear iron cluster that is perturbed by substrate binding in myo-inositol oxygenase". Biochemistry. 45 (17): 5393–5401. doi:10.1021/bi0519607. ISSN 0006-2960. PMID 16634620.
- ↑ Xing, Gang; Diao, Yinghui; Hoffart, Lee M.; Barr, Eric W.; Prabhu, K. Sandeep; Arner, Ryan J.; Reddy, C. Channa; Krebs, Carsten; Bollinger, J. Martin (2006-04-18). "Evidence for C-H cleavage by an iron-superoxide complex in the glycol cleavage reaction catalyzed by myo-inositol oxygenase". Proceedings of the National Academy of Sciences of the United States of America. 103 (16): 6130–6135. doi:10.1073/pnas.0508473103. ISSN 0027-8424. PMC 1458843
 . PMID 16606846.
. PMID 16606846. - 1 2 Croze, Marine L.; Soulage, Christophe O. (2013-10-01). "Potential role and therapeutic interests of myo-inositol in metabolic diseases". Biochimie. 95 (10): 1811–1827. doi:10.1016/j.biochi.2013.05.011. ISSN 1638-6183. PMID 23764390.
- ↑ Cohen RA, MacGregor LC, Spokes KC, Silva P, Epstein FH (Oct 1990). "Effect of myo-inositol on renal Na-K-ATPase in experimental diabetes". Metabolism. 39 (10): 1026–32. doi:10.1016/0026-0495(90)90161-5. PMID 2170818.
- 1 2 Tominaga, Tatsuya; Dutta, Rajesh K.; Joladarashi, Darukeshwara; Doi, Toshio; Reddy, Janardan K.; Kanwar, Yashpal S. (2016-01-15). "Transcriptional and Translational Modulation of myo-Inositol Oxygenase (Miox) by Fatty Acids: IMPLICATIONS IN RENAL TUBULAR INJURY INDUCED IN OBESITY AND DIABETES". The Journal of Biological Chemistry. 291 (3): 1348–1367. doi:10.1074/jbc.M115.698191. ISSN 1083-351X. PMC 4714220
 . PMID 26578517.
. PMID 26578517. - ↑ Gaut, Joseph P.; Crimmins, Dan L.; Ohlendorf, Matt F.; Lockwood, Christina M.; Griest, Terry A.; Brada, Nancy A.; Hoshi, Masato; Sato, Bryan; Hotchkiss, Richard S. (2014-05-01). "Development of an immunoassay for the kidney-specific protein myo-inositol oxygenase, a potential biomarker of acute kidney injury". Clinical Chemistry. 60 (5): 747–757. doi:10.1373/clinchem.2013.212993. ISSN 1530-8561. PMC 4128578
 . PMID 24486646.
. PMID 24486646. - ↑ Moon, Tae Seok; Yoon, Sang-Hwal; Lanza, Amanda M.; Roy-Mayhew, Joseph D.; Prather, Kristala L. Jones (2009-02-01). "Production of glucaric acid from a synthetic pathway in recombinant Escherichia coli". Applied and Environmental Microbiology. 75 (3): 589–595. doi:10.1128/AEM.00973-08. ISSN 1098-5336. PMC 2632142
 . PMID 19060162.
. PMID 19060162. - ↑ Shiue, Eric; Prather, Kristala L. J. (2014-03-01). "Improving D-glucaric acid production from myo-inositol in E. coli by increasing MIOX stability and myo-inositol transport". Metabolic Engineering. 22: 22–31. doi:10.1016/j.ymben.2013.12.002. ISSN 1096-7184. PMID 24333274.
External links
- Inositol Oxygenase at the US National Library of Medicine Medical Subject Headings (MeSH)