Derrubone
 | |
| Names | |
|---|---|
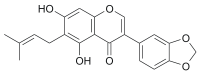
| IUPAC name
3-(1,3-Benzodioxol-5-yl)-5,7-dihydroxy-6-(3-methylbut-2-enyl)chromen-4-one | |
| Other names
5,7-Dihydroxy-3',4'-methylenedioxy-6-prenylisoflavone | |
| Identifiers | |
| 22044-58-2 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL412010 |
| ChemSpider | 4709237 |
| PubChem | 5810067 |
| |
| |
| Properties | |
| C21H18O6 | |
| Molar mass | 366.37 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Derrubone is a prenylated isoflavone, a type of flavonoid. It was originally isolated from the Indian tree Derris robusta.[1] Recent research indicates that it acts as an inhibitor of Hsp90 to its function as a chaperone protein.[2]
References
- ↑ East AJ, Ollis WD, Wheeler RE (1969). "Natural occurrence of 3-aryl-4-hydroxycoumarins. Part I. Phytochemical examination of Derris robusta(roxb.) benth.". J. Chem. Soc. C. 3: 365–74. doi:10.1039/J39690000365.
- ↑ Hadden MK, Galam L, Gestwicki JE, Matts RL, Blagg BS (December 2007). "Derrubone, an inhibitor of the Hsp90 protein folding machinery". J. Nat. Prod. 70 (12): 2014–8. doi:10.1021/np070190s. PMID 18020309.
This article is issued from Wikipedia - version of the 5/22/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.