DNA adenine methyltransferase identification
DamID[1] (DNA adenine methyltransferase identification) is a molecular biology protocol used to map the binding sites of DNA- and chromatin-binding proteins in eukaryotes. DamID identifies binding sites by expressing the proposed DNA-binding protein as a fusion protein with DNA methyltransferase. Binding of the protein of interest to DNA localizes the methyltransferase in the region of the binding site. Adenosine methylation does not occur naturally in eukaryotes and therefore adenine methylation in any region can be concluded to have been caused by the fusion protein, implying the region is located near a binding site. DamID is an alternate method to ChIP-on-chip.
Description of the method
Principle
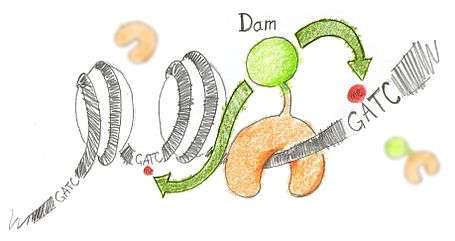
N6-methyladenine (m6A) is the product of the addition of a methyl group (CH3) at position 6 of the adenine. This modified nucleotide is absent from the vast majority of eukaryotes, but is widespread in bacterial genomes,[2] as part of the restriction modification or DNA repair systems. In Escherichia coli, adenine methylation is catalyzed by the adenine methyltransferase Dam (DNA adenine methyltransferase), which catalyses adenine methylation exclusively in the palindromic sequence GATC. Ectopic expression of Dam in eukaryotic cells leads to methylation of adenine in GATC sequences without any other noticeable side effect.
Based on this, DamID consists in fusing Dam to a protein of interest (usually a protein that interacts with DNA such as transcription factors) or a chromatin component. The protein of interest thus targets Dam to its cognate in vivo binding site, resulting in the methylation of neighboring GATCs. The presence of m6A, coinciding with the binding sites of the proteins of interest, is revealed by methyl PCR.
Methyl PCR (mePCR)
In this assay the genome is digested by DpnI, which cuts only methylated GATCs. Double-stranded adapters with a known sequence are then ligated to the ends generated by DpnI. A PCR with primers matching the adaptors is then carried out, leading to the specific amplification of genomic fragments flanked by methylated GATCs. In practice, ligation products are digested by DpnII prior PCR amplification. This enzyme cuts non-methylated GATCs, ensuring that only fragments flanked by consecutive methylated GATCs are amplified.
Specificities of DamID versus ChIP (Chromatin Immuno-Precipitation)
ChIP is an alternative method to assay protein binding at specific loci of the genome. Unlike ChIP, DamID does not require a specific antibody against the protein of interest. On the one hand, this allows to map proteins for which no such antibody is available. On the other hand, this makes it impossible to specifically map posttranslationally modified proteins.
Another fundamental difference is that ChIP assays where the protein of interests is at a given time, whereas DamID assays where it has been. The reason is that m6A stays in the DNA after the Dam fusion protein goes away. For proteins that are either bound or unbound on their target sites this does not change the big picture. However, this can lead to strong differences in the case of proteins that slide along the DNA (e.g. RNA polymerase).
Known biases and technical issues
Plasmid methylation bias
Depending on how the experiment is carried out, DamID can be subject to plasmid methylation biases. Because plasmids are usually amplified in E. coli where Dam is naturally expressed, they are methylated on every GATC. In transient transfection experiments, the DNA of those plasmids is recovered along with the DNA of the transfected cells, meaning that fragments of the plasmid are amplified in the mePCR. Every sequence of the genome that shares homology or identity with the plasmid may thus appear to be bound by the protein of interest. In particular, this is true of the open reading frame of the protein of interest, which is present in both the plasmid and the genome. In microarray experiments, this bias can be used to ensure that the proper material was hybridized.
Apoptosis
Apoptotic cells degrade their DNA in a characteristic nucleosome ladder pattern. This generates DNA fragments that can be ligated and amplified during the DamID procedure (van Steensel laboratory, unpublished observations). The influence of these nucleosomal fragments on the binding profile of a protein is not known.
Resolution
The resolution of DamID is a function of the availability of GATC sequences in the genome. A protein can only be mapped within two consecutive GATC sites. The median spacing between GATC fragments is 205 bp in Drosophila (FlyBase release 5), 260 in mouse (Mm9), and 460 in human (HG19).
References
- ↑ van Steensel B, Henikoff S (April 2000). "Identification of in vivo DNA targets of chromatin proteins using tethered dam methyltransferase". Nat. Biotechnol. 18 (4): 424–8. doi:10.1038/74487. PMID 10748524.
- ↑ Brooks J. E.; Roberts R. J. (1982). "Modification profiles of bacterial genomes". Nucleic Acids Res. 10 (3): 913–34. doi:10.1093/nar/10.3.913. PMC 326211
 . PMID 6278441.
. PMID 6278441.
External links
- Vogel MJ, Peric-Hupkes D, van Steensel B (2007). "Detection of in vivo protein-DNA interactions using DamID in mammalian cells". Nat Protoc. 2 (6): 1467–78. doi:10.1038/nprot.2007.148. PMID 17545983.
- Greil F, Moorman C, van Steensel B (2006). "DamID: mapping of in vivo protein-genome interactions using tethered DNA adenine methyltransferase". Meth. Enzymol. 410: 342–59. doi:10.1016/S0076-6879(06)10016-6. PMID 16938559.
- Frequently asked questions about DamID
- Germann S, Juul-Jensen T, Letarnec B, Gaudin V (October 2006). "DamID, a new tool for studying plant chromatin profiling in vivo, and its use to identify putative LHP1 target loci". Plant J. 48 (1): 153–63. doi:10.1111/j.1365-313X.2006.02859.x. PMID 16972870.