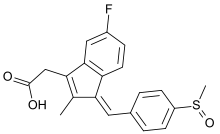
Sulindac
 | |
| Clinical data | |
|---|---|
| Trade names | Clinoril |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681037 |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | M01AB02 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Approximately 90% (Oral) |
| Metabolism | ? |
| Biological half-life | 7.8 hours, metabolites up to 16.4 hours |
| Excretion | Renal (50%) and fecal (25%) |
| Identifiers | |
| |
| CAS Number |
38194-50-2 |
| PubChem (CID) | 1548887 |
| IUPHAR/BPS | 5425 |
| DrugBank |
DB00605 |
| ChemSpider |
1265915 |
| UNII |
184SNS8VUH |
| KEGG |
D00120 |
| ChEBI |
CHEBI:9352 |
| ChEMBL |
CHEMBL15770 |
| PDB ligand ID | SUZ (PDBe, RCSB PDB) |
| ECHA InfoCard | 100.048.909 |
| Chemical and physical data | |
| Formula | C20H17FO3S |
| Molar mass | 356.412 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 182 to 185 °C (360 to 365 °F) (decomp.) |
| |
| |
| | |
Sulindac is a non-steroidal anti-inflammatory drug of the arylalkanoic acid class that is marketed in the UK & U.S. by Merck as Clinoril. Imbaral (not mebaral) is another name for this drug.
Uses
Like other NSAIDs, it is useful in the treatment of acute or chronic inflammatory conditions. Sulindac is a prodrug, derived from sulfinylindene, that is converted in the body to the active NSAID. More specifically, the agent is converted by liver enzymes to a sulfide that is excreted in the bile and then reabsorbed from the intestine. This is thought to help maintain constant blood levels with reduced gastrointestinal side effects. Some studies have shown sulindac to be relatively less irritating to the stomach than other NSAIDs except for drugs of the COX-2 inhibitor class . The exact mechanism of its NSAID properties is unknown, but it is thought to act on enzymes COX-1 and COX-2, inhibiting prostaglandin synthesis.
Its usual dosage is 150-200 milligrams twice per day, with food. It should not be used by persons with a history of major allergic reactions (urticaria or anaphylaxis) to aspirin or other NSAIDs, and should be used with caution by persons having pre-existing peptic ulcer disease. Sulindac is much more likely than other NSAIDs to cause damage to the liver or pancreas However it is less likely to cause kidney damage than other NSAIDs.
Sulindac seems to have a property, independent of COX-inhibition, of reducing the growth of polyps and precancerous lesions in the colon, especially in association with familial adenomatous polyposis, and may have other anti-cancer properties.[1][2]
Sulindac is an effective tocolytic and may be used in the treatment of preterm labor. In common with other NSAIDs, sulindac is currently being investigated for its role in the treatment of Alzheimer's disease.
Since it was found that the sulfoxide functional group can be reduced by methionine sulfoxide reductase A (MsrA), a possible anti-oxidative capability is being discussed.
Litigation
In September 2010 a federal jury in New Hampshire awarded $21 million to Karen Bartlett, a woman who developed Stevens–Johnson syndrome/Toxic epidermal necrolysis as a result of taking a generic brand of sulindac manufactured by Mutual Pharmaceuticals for her shoulder pain. Ms. Bartlett suffered severe injuries including the loss of over 60% of her surface skin and permanent near-blindness. The case had been appealed to the United States Supreme Court, where the main issue was whether federal law preempts Ms. Bartlett's claim.[3] On June 24, 2013, the Supreme Court ruled 5-4 in favor of Mutual Pharmaceuticals, throwing out the earlier $21 million jury verdict.[4]
Cite to:
Bartlett v. Mut. Pharm. Co., Inc., 678 F.3d 30, 34 (1st Cir. 2012).
Synthesis
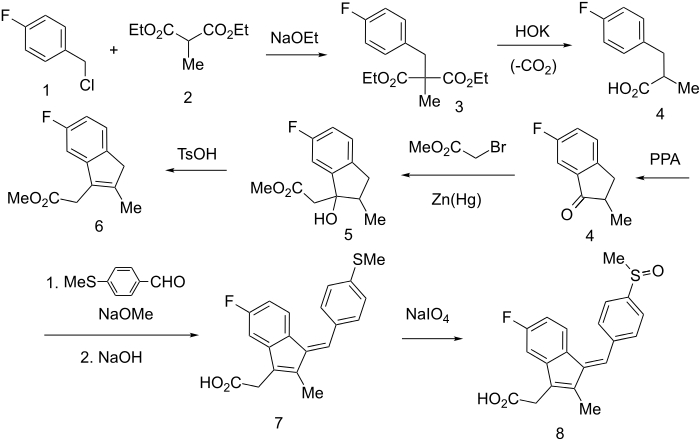
Rxn of p-fluorobenzyl chloride (1) with the anion of diethylmethyl malonate (2) gives intermediate diester (3), saponification of which and subsequent decarboxylation leads to 4. {Alternatively it can be formed by Perkin reaction between p-fluorobenzaldehyde and propionic anhydride in the presence of NaOAc, followed by catalytic hydrogenation of the olefinic bond using a palladium on carbon catalyst.}
Polyphosphoric acid (PPA) cyclization leads to 5-fluoro-2-methyl-3-indanone (4). A Reformatsky reaction with zinc amalgam and bromoacetic ester leads to carbinol (5), which is then dehydrated with tosic acid to indene 6. {Alternatively ths step can be performed in a Knoevenagel condensation with cyanoacetic acid, which is then further decarboxylated.}
The active methylene group is condensed with p-methylthiobenzaldehyde, using sodium methoxide as catalyst, and then saponified to give Z (7) which in turn oxidized with sodium metaperiodate to sulfoxide 8, the antiinflammatory agent sulindac.
References
- ↑ Scheper MA, Nikitakis NG, Chaisuparat R, Montaner S, Sauk JJ (March 2007). "Sulindac induces apoptosis and inhibits tumor growth in vivo in head and neck squamous cell carcinoma". Neoplasia. 9 (3): 192–9. doi:10.1593/neo.06781. PMC 1838577
 . PMID 17401459.
. PMID 17401459. - ↑ Shiff SJ, Qiao L, Tsai LL, Rigas B (July 1995). "Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells". J. Clin. Invest. 96 (1): 491–503. doi:10.1172/JCI118060. PMC 185223
 . PMID 7615821.
. PMID 7615821. - ↑ Thomas, Katie. "Justices to Take Up Case on Generic Drug Markers' Liability". New York Times. Retrieved 4 March 2013.
- ↑ Kendall, Brent. "Supreme Court Again Limits Product-Liability Suits on Generic Drugs". Wall Street Journal. Retrieved 24 June 2013.
- ↑ Shuman, R. F.; Pines, S. H.; Shearin, W. E.; Czaja, R. F.; Abramson, N. L.; Tull, R. (1977). "A sterically efficient synthesis of (Z)-5-fluoro-2-methyl-1-(p-methylthiobenzylidene)-3-indenylacetic acid and its S-oxide, sulindac". The Journal of Organic Chemistry. 42 (11): 1914. doi:10.1021/jo00431a019.
- ↑ R.B. Greenwald, E.B. Witzel, DE 2039426 (1971).
- ↑ J.B. Conn, D.F. Hinkley,U.S. Patent 3,647,858 (1972).
- ↑ R.B. Greenwald, H. Jones, U.S. Patent 3,654,349 (1972).