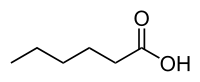
Hexanoic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Hexanoic acid | |
| Other names
Caproic acid; n-Caproic acid; C6:0 (Lipid numbers) | |
| Identifiers | |
| 142-62-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:30776 |
| ChEMBL | ChEMBL14184 |
| ChemSpider | 8552 |
| ECHA InfoCard | 100.005.046 |
| KEGG | C01585 |
| PubChem | 8892 |
| UNII | 1F8SN134MX |
| |
| |
| Properties | |
| C6H12O2 | |
| Molar mass | 116.16 g·mol−1 |
| Appearance | Oily liquid[1] |
| Odor | goat-like |
| Density | 0.929 g/cm3[2] |
| Melting point | −3.4 °C (25.9 °F; 269.8 K)[1] |
| Boiling point | 205.8 °C (402.4 °F; 478.9 K)[1] |
| 1.082 g/100 mL[1] | |
| Solubility | soluble in ethanol, ether |
| Acidity (pKa) | 4.88 |
| Refractive index (nD) |
1.4170 |
| Viscosity | 3.1 mP |
| Hazards | |
| NFPA 704 | |
| Flash point | 103 °C (217 °F; 376 K)[2] |
| 380 °C (716 °F; 653 K) | |
| Explosive limits | 1.3-9.3% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
3000 mg/kg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hexanoic acid (caproic acid) is the carboxylic acid derived from hexane with the general formula C5H11COOH. It is a colorless oily liquid with an odor that is fatty, cheesy, waxy, and like that of goats[1] or other barnyard animals. It is a fatty acid found naturally in various animal fats and oils, and is one of the chemicals that give the decomposing fleshy seed coat of the ginkgo its characteristic unpleasant odor.[3] It is also one of the components of vanilla. The primary use of hexanoic acid is in the manufacture of its esters for artificial flavors, and in the manufacture of hexyl derivatives, such as hexylphenols.[1]
The salts and esters of this acid are known as hexanoates or caproates.
Two other acids are named after goats: caprylic (C8) and capric (C10). Along with hexanoic acid, these total 15% in goat milk fat.
Caproic, caprylic, and capric acids (capric is a crystal- or wax-like substance, whereas the other two are mobile liquids) are not only used for the formation of esters, but also commonly used "neat" in: butter, milk, cream, strawberry, bread, beer, nut, and other flavors.
