Caesium sulfate
 | |
| Names | |
|---|---|
| Other names
Cesium sulfate | |
| Identifiers | |
| 10294-54-9 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 23482 |
| ECHA InfoCard | 100.030.589 |
| EC Number | 233-662-6 |
| UNII | 8D6R91CS62 |
| |
| |
| Properties | |
| Cs2SO4 | |
| Molar mass | 361.87 g/mol |
| Density | 4.243 g/cm3, solid |
| Melting point | 1,010 °C (1,850 °F; 1,280 K) |
| 167 g/100 ml (0 °C) | |
| Solubility | insoluble in ethanol, acetone |
| Hazards | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
2830 mg/kg (oral, rat)[2] |
| Related compounds | |
| Other anions |
Caesium hydrogensulfate |
| Other cations |
Lithium sulfate Sodium sulfate Potassium sulfate Rubidium sulfate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Caesium sulfate (cesium sulfate) is the inorganic compound and salt with the formula Cs2SO4. It is a white water-soluble solid that is used to prepare dense aqueous solutions for use in isopycnic (or "density-gradient") centrifugation. It is isostructural with potassium salt.[3]
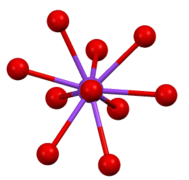 Coordination sphere of one of two types of Cs+ site in Cs2SO4.
Coordination sphere of one of two types of Cs+ site in Cs2SO4. Environment of sulfate anion in β-Cs2SO4.
Environment of sulfate anion in β-Cs2SO4.
References
- ↑ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B-92. ISBN 0-8493-0462-8..
- ↑ http://chem.sis.nlm.nih.gov/chemidplus/rn/10294-54-9
- ↑ Nord, A.G. "The crystal structure of cesium sulfate, beta-Cs2SO4" Acta Chemica Scandinavica, Series A: 1976, 30, p198.
External links
| Salts and esters of the sulfate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2SO4 | He | ||||||||||||||||||
| Li2SO4 | BeSO4 | B | esters ROSO3− (RO)2SO2 |
(NH4)2SO4 N2H6SO4 (NH3OH)2SO4 |
O | F | Ne | ||||||||||||
| Na2SO4 NaHSO4 |
MgSO4 | Al2(SO4)3 Al2SO4(OAc)4 |
Si | P | SO42− | Cl | Ar | ||||||||||||
| K2SO4 KHSO4 |
CaSO4 | Sc2(SO4)3 | Ti(SO4)2 TiOSO4 |
V2(SO4)3 VOSO4 |
CrSO4 Cr2(SO4)3 |
MnSO4 | FeSO4 Fe2(SO4)3 |
CoSO4, Co2(SO4)3 |
NiSO4 | CuSO4 | ZnSO4 | Ga2(SO4)3 | Ge | As | Se | Br | Kr | ||
| RbHSO4 Rb2SO4 |
SrSO4 | Y2(SO4)3 | Zr(SO4)2 | Nb | Mo | Tc | Ru | Rh | PdSO4 | Ag2SO4 | CdSO4 | In2(SO4)3 | SnSO4 | Sb2(SO4)3 | Te | I | Xe | ||
| Cs2SO4 | BaSO4 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2SO4, HgSO4 |
Tl2SO4 | PbSO4 | Bi2(SO4)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce2(SO4)3 Ce(SO4)2 |
Pr2(SO4)3 | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb2(SO4)3 | Lu | |||||
| Ac | Th | Pa | U(SO4)2 UO2SO4 |
Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
This article is issued from Wikipedia - version of the 11/10/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.