Benzilic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Hydroxy(diphenyl)acetic acid | |
| Other names
α,α-Diphenyl-α-hydroxyacetic acid, α,α-Diphenylglycolic acid, α-Hydroxydiphenyl acetic acid, 2,2-Diphenyl-2-hydroxyacetic acid, 2-Hydroxy-2,2-diphenylacetic acid, Diphenyl glycolic acid, Hydroxydiphenyl acetic acid | |
| Identifiers | |
| 76-93-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:39414 |
| ChEMBL | ChEMBL578171 |
| ChemSpider | 6220 |
| ECHA InfoCard | 100.000.904 |
| PubChem | 6463 |
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.25 g·mol−1 |
| Appearance | white solid |
| Density | 1.08 g/cm3 |
| Melting point | 150 to 152 °C (302 to 306 °F; 423 to 425 K) |
| Boiling point | 180 °C (356 °F; 453 K) (17.3 hPa) |
| 2 g/l (20 °C) | |
| Hazards | |
| R-phrases | R22 |
| S-phrases | S23, S24, S25, S28, S36, S37, S45 |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
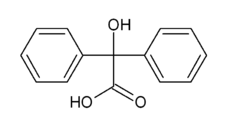
Benzilic acid is an organic compound with formula C
14H
12O
3 or (C
6H
5)2(HO)C(COOH). It is a white crystalline aromatic acid, soluble in many primary alcohols.
Preparation
Benzilic acid can be prepared by heating mixture of benzil, ethanol and potassium hydroxide.
Another preparation, performed by Liebig in 1838, is the dimerization of benzaldehyde, to benzil, which is transformed to the product by the benzilic acid rearrangement reaction.[1]
Uses
Benzilic acid is used in the manufacture of glycollate pharmaceuticals including Clidinium, Dilantin, and Flutropium, which are antagonists of the muscarinic acetylcholine receptors.
It is used in manufacture of the incapacitating agent 3-quinuclidinyl benzilate (BZ) and is regulated by the Chemical Weapons Convention. It is also monitored by law enforcement agencies of many countries, because of its use in the manufacture in hallucinogenic drugs.[2]
References
- ↑ Liebig, J. (1838). "Ueber Laurent's Theorie der organischen Verbindungen". Annalen der Chemie. 25: 1–31. doi:10.1002/jlac.18380250102.
- ↑ "Nerve Agent Precursors: Benzilic acid and Methyl Benzilate", Factsheets on Chemical and Biological Warfare Agents, Chemical precursors.
External links
- Safety MSDS data
- Solubility in alcohols
- Converting Benzaldehyde to Benzilic Acid
- Synthesis of Benzilic Acid
