Becquerel
| Becquerel | |
|---|---|
| Unit system | SI derived unit |
| Unit of | Radioactivity |
| Symbol | Bq |
| Named after | Henri Becquerel |
| In SI base units | s−1 |
The becquerel (symbol Bq) (pronounced: /ˈbɛkərɛl/ BEK-ə-rel) is the SI derived unit of radioactivity. One becquerel is defined as the activity of a quantity of radioactive material in which one nucleus decays per second. The becquerel is therefore equivalent to an inverse second, s−1. The becquerel is named after Henri Becquerel, who shared a Nobel Prize with Pierre Curie and Marie Curie in 1903 for their work in discovering radioactivity.[1]
Capitalization
As with every International System of Units (SI) unit named for a person, the first letter of its symbol is uppercase (Bq). However, when an SI unit is spelled out in English, it should always begin with a lowercase letter (becquerel)—except in a situation where any word in that position would be capitalized, such as at the beginning of a sentence or in material using title case.[2]
Definition
1 Bq = 1 s−1
A special name was introduced for the reciprocal second (s−1) to represent radioactivity to avoid potentially dangerous mistakes with prefixes. For example, 1 µs−1 could be taken to mean 106 disintegrations per second: 1·(10−6 s)−1 = 106 s−1.[3] Other names considered were hertz (Hz), a special name already in use for the reciprocal second, and fourier (Fr).[3] The hertz is now only used for periodic phenomena.[4] Whereas 1 Hz is 1 cycle per second, 1 Bq is 1 aperiodic radioactivity event per second.
The gray (Gy) and the becquerel (Bq) were introduced in 1975.[5] Between 1953 and 1975, absorbed dose was often measured in rads. Decay activity was measured in curies before 1946 and often in rutherfords between 1946[6] and 1975.
Prefixes
Like any SI unit, Bq can be prefixed; commonly used multiples are kBq (kilobecquerel, 103 Bq), MBq (megabecquerel, 106 Bq, equivalent to 1 rutherford), GBq (gigabecquerel, 109 Bq), TBq (terabecquerel, 1012 Bq), and PBq (petabecquerel, 1015 Bq). For practical applications, 1 Bq is a small unit; therefore, the prefixes are common. For example, the roughly 0.0169 g of potassium-40 present in a typical human body produces approximately 266,000 disintegrations per minute, which equates to about 4,400 disintegrations per second or 4.4 kBq of activity.[7] The global inventory of carbon-14 is estimated to be 8.5×1018 Bq (8.5 EBq, 8.5 exabecquerel).[8] The nuclear explosion in Hiroshima (An explosion of 16 kt or 67 TJ) is estimated to have produced 8×1024 Bq (8 YBq, 8 yottabecquerel).[9]
Relationship to the curie
The becquerel succeeded the curie (Ci),[10] an older, non-SI unit of radioactivity based on the activity of 1 gram of radium-226. The curie is defined as 3.7·1010 s−1, or 37 GBq.[3]
Conversion factors:
- 1 Ci = 3.7×1010 Bq = 37 GBq
- 1 μCi = 37,000 Bq = 37 kBq
- 1 Bq = 2.7×10−11 Ci = 2.7×10−5 µCi
- 1 MBq = 0.027 mCi
Calculation of radioactivity
For a given mass (in grams) of an isotope with atomic mass (in g/mol) and a half-life of (in s), the amount of radioactivity can be calculated using:
With =6.022 141 79(30)×1023 mol−1, the Avogadro constant.
Since m/ma is the number of moles (n), the amount of radioactivity can be calculated by:
For instance, one gram of potassium contains 0.000117 gram of 40K (all other naturally occurring isotopes are stable) that has a of 1.277×109 years = 4.030×1016 s,[11] and has an atomic mass of 39.964 g/mol,[12] so the radioactivity is 30 Bq.
Radiation-related quantities
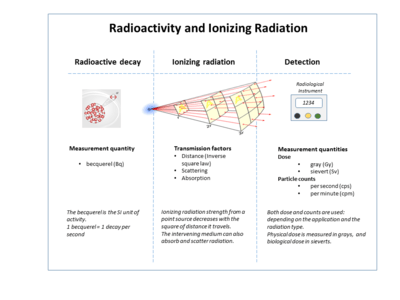
The following table shows radiation quantities in SI and non-SI units.
| Quantity | Name | Symbol | Unit | Year |
|---|---|---|---|---|
| Exposure (X) | roentgen | R | esu / 0.001293 g of air | 1928 |
| Absorbed dose (D) | erg·g−1 | 1950 | ||
| rad | rad | 100 erg·g−1 | 1953 | |
| gray | Gy | J·kg−1 | 1974 | |
| Activity (A) | curie | Ci | 3.7 × 1010 s−1 | 1953 |
| becquerel | Bq | s−1 | 1974 | |
| Dose equivalent (H) | roentgen equivalent man | rem | 100 erg·g−1 | 1971 |
| sievert | Sv | J·kg−1 | 1977 | |
| Fluence (Φ) | (reciprocal area) | cm−2 or m−2 | 1962 |
See also
- Background radiation
- Banana equivalent dose
- Counts per minute
- Ionizing radiation
- Orders of magnitude (radiation)
- Radiation poisoning
- Relative Biological Effectiveness
- Rem (unit)
- Rutherford (unit)
- Sievert (biological dose equivalent of radiation)
References
- ↑ "BIPM - Becquerel". BIPM. Retrieved 2012-10-24.
- ↑ "SI Brochure: The International System of Units (SI)". SI Brochure (8 ed.). BIPM. 2014.
- 1 2 3 Allisy, A. (1995), "From the curie to the becquerel", Metrologia, 32 (6): 467–479, Bibcode:1995Metro..31..467A, doi:10.1088/0026-1394/31/6/006
- ↑ "BIPM - Table 3". BIPM. Retrieved 2015-07-19.
(d) The hertz is used only for periodic phenomena, and the becquerel is used only for stochastic processes in activity referred to a radionuclide.
- ↑ Harder, D (1976), "[The new radiologic units of measurement gray and becquerel (author's translation from the German original)]", Röntgen-Blätter, 29 (1): 49–52, PMID 1251122.
- ↑ Lind, SC (1946), "New units for the measurement of radioactivity", Science, 103 (2687): 761–762, Bibcode:1946Sci...103..761L, doi:10.1126/science.103.2687.761-a, PMID 17836457.
- ↑ Radioactive human body — Harvard University Natural Science Lecture Demonstrations - Accessed October 2013
- ↑ G.R. Choppin, J.O.Liljenzin, J. Rydberg, "Radiochemistry and Nuclear Chemistry", 3rd edition, Butterworth-Heinemann, 2002. ISBN 978-0-7506-7463-8.
- ↑ Michael J. Kennish, Pollution Impacts on Marine Biotic Communities , CRC Press, 1998, p. 74. ISBN 978-0-8493-8428-8.
- ↑ It was adopted by the BIPM in 1975, see resolution 8 of the 15th CGPM meeting
- ↑ "Table of Isotopes decay data". Lund University. 1990-06-01. Retrieved 2014-01-12.
- ↑ "Atomic Weights and Isotopic Compositions for All Elements". NIST. Retrieved 2014-01-12.
External links
| Look up becquerel in Wiktionary, the free dictionary. |
- Derived units on the International Bureau of Weights and Measures (BIPM) web site