Electrochemical cell
An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt cell meant for consumer use. This type of device is known as a single Galvanic cell. A battery consists of two or more cells, connected in either parallel or series pattern.[1]
Half-cells
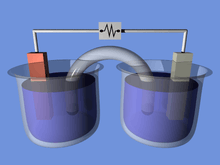
An electrochemical cell consists of two half-cells. Each half-cell consists of an electrode and an electrolyte. The two half-cells may use the same electrolyte, or they may use different electrolytes. The chemical reactions in the cell may involve the electrolyte, the electrodes, or an external substance (as in fuel cells that may use hydrogen gas as a reactant). In a full electrochemical cell, species from one half-cell lose electrons (oxidation) to their electrode while species from the other half-cell gain electrons (reduction) from their electrode.
A Salt Bridge (e.g., filter paper soaked in KNO3, NaCl, or some other electrolyte) is often employed to provide ionic contact between two half-cells with different electrolytes, yet prevent the solutions from mixing and causing unwanted side reactions. An alternative to a salt bridge is to allow direct contact (and mixing) between the two half-cells, for example in simple electrolysis of water.
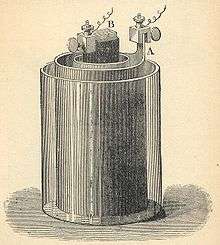
As electrons flow from one half-cell to the other through an external circuit, a difference in charge is established. If no ionic contact were provided, this charge difference would quickly prevent the further flow of electrons. A salt bridge allows the flow of negative or positive ions to maintain a steady-state charge distribution between the oxidation and reduction vessels, while keeping the contents otherwise separate. Other devices for achieving separation of solutions are porous pots and gelled solutions. A porous pot is used in the Bunsen cell (right).
Equilibrium reaction
Each half-cell has a characteristic voltage. Various choices of substances for each half-cell give different potential differences. Each reaction is undergoing an equilibrium reaction between different oxidation states of the ions: When equilibrium is reached, the cell cannot provide further voltage. In the half-cell that is undergoing oxidation, the closer the equilibrium lies to the ion/atom with the more positive oxidation state the more potential this reaction will provide. Likewise, in the reduction reaction, the closer the equilibrium lies to the ion/atom with the more negative oxidation state the higher the potential.
Cell potential
The cell potential can be predicted through the use of electrode potentials (the voltages of each half-cell). These half-cell potentials are defined relative to the assignment of 0 volts to the standard hydrogen electrode (SHE). (See table of standard electrode potentials). The difference in voltage between electrode potentials gives a prediction for the potential measured. When calculating the difference in voltage, one must first rewrite the half-cell reaction equations to obtain a balanced oxidation-reduction equation.
- Reverse the reduction reaction with the smallest potential ( to create an oxidation reaction/ overall positive cell potential)
- Half-reactions must be multiplied by integers to achieve electron balance.
Note that the cell potential does not change when the reaction is multiplied by a constant.
Cell potentials have a possible range of roughly zero to 6 volts. Cells using water-based electrolytes are usually limited to cell potentials less than about 2.5 volts, because the very powerful oxidizing and reducing agents that would be required to produce a higher cell potential tend to react with the water. Higher cell potentials are possible with cells using other solvents instead of water. For instance, lithium cells with a voltage of 3 volts are commonly available.
The cell potential depends on the concentration of the reactants, as well as their type. As the cell is discharged, the concentration of the reactants decreases, and the cell potential also decreases.