Biofilm

Aggregate of microorganisms in which cells that are frequently embedded within a self-produced matrix of extracellular polymeric substance (EPS) adhere to each other and/or to a surface.
Note 1: A biofilm is a system that can be adapted internally to environmental conditions by its inhabitants.
Note 2: The self-produced matrix of extracellular polymeric substance, which is also referred to as slime, is a polymeric conglomeration generally composed of extracellular biopolymers in various structural forms.[1]
A biofilm is any group of microorganisms in which cells stick to each other and often these cells adhere to a surface. These adherent cells are frequently embedded within a self-produced matrix of extracellular polymeric substance (EPS). Biofilm extracellular polymeric substance, which is also referred to as slime (although not everything described as slime is a biofilm), is a polymeric conglomeration generally composed of extracellular DNA, proteins, and polysaccharides. Biofilms may form on living or non-living surfaces and can be prevalent in natural, industrial and hospital settings.[2][3] The microbial cells growing in a biofilm are physiologically distinct from planktonic cells of the same organism, which, by contrast, are single-cells that may float or swim in a liquid medium.
Microbes form a biofilm in response to many factors, which may include cellular recognition of specific or non-specific attachment sites on a surface, nutritional cues, or in some cases, by exposure of planktonic cells to sub-inhibitory concentrations of antibiotics.[4][5] When a cell switches to the biofilm mode of growth, it undergoes a phenotypic shift in behavior in which large suites of genes are differentially regulated.[6]
Formation
Formation of a biofilm begins with the attachment of free-floating microorganisms to a surface. While still not fully understood, it is thought that the first colonists of a biofilm adhere to the surface initially through weak, reversible adhesion via van der Waals forces and hydrophobic effects.[7][8] If the colonists are not immediately separated from the surface, they can anchor themselves more permanently using cell adhesion structures such as pili. Hydrophobicity also plays an important role in determining the ability of bacteria to form biofilms, as those with increased hydrophobicity have reduced repulsion between the extracellular matrix and the bacterium.[9]
Some species are not able to attach to a surface on their own but are instead able to anchor themselves to the matrix or directly to earlier colonists. It is during this colonization that the cells are able to communicate via quorum sensing (QS) using products such as N-acyl homoserine lactone (AHL). Some bacteria are unable to form biofilms as successfully due to their limited motility. Non-motile bacteria cannot recognize the surface or aggregate together as easily as motile bacteria.[9] Once colonization has begun, the biofilm grows through a combination of cell division and recruitment. Polysaccharide matrices typically enclose bacterial biofilms. In addition to the polysaccharides, these matrices may also contain material from the surrounding environment, including but not limited to minerals, soil particles, and blood components, such as erythrocytes and fibrin.[9] The final stage of biofilm formation is known as dispersion, and is the stage in which the biofilm is established and may only change in shape and size.
The development of a biofilm may allow for an aggregate cell colony (or colonies) to be increasingly resistant to antibiotics. Cell-cell communication or quorum sensing has been shown to be involved in the formation of biofilm in several bacterial species.[10]
Development
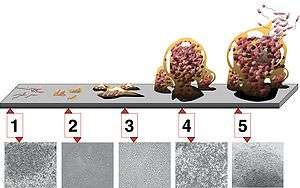
There are five stages of biofilm development (see illustration at right):[11]
- Initial attachment.
- Irreversible attachment.
- Maturation I.
- Maturation II.
- Dispersion.
Dispersal
Dispersal of cells from the biofilm colony is an essential stage of the biofilm life cycle. Dispersal enables biofilms to spread and colonize new surfaces. Enzymes that degrade the biofilm extracellular matrix, such as dispersin B and deoxyribonuclease, may play a role in biofilm dispersal.[12][13] Biofilm matrix degrading enzymes may be useful as anti-biofilm agents.[14][15] Recent evidence has shown that a fatty acid messenger, cis-2-decenoic acid, is capable of inducing dispersion and inhibiting growth of biofilm colonies. Secreted by Pseudomonas aeruginosa, this compound induces cyclo heteromorphic cells in several species of bacteria and the yeast Candida albicans.[16] Nitric oxide has also been shown to trigger the dispersal of biofilms of several bacteria species[17][18] at sub-toxic concentrations. Nitric oxide has the potential for the treatment of patients that suffer from chronic infections caused by biofilms.[19]
It is generally assumed that cells dispersed from biofilms immediately go into the planktonic growth phase. However, recent studies have shown that the physiology of dispersed cells from Pseudomonas aeruginosa biofilms is highly different from those of planktonic and biofilm cells.[20][21] Hence, the dispersal process is a unique stage during the transition from biofilm to planktonic lifestyle in bacteria. Dispersed cells are found to be highly virulent against macrophages and Caenorhabditis elegans, but highly sensitive towards iron stress, as compared with planktonic cells.[20]
Properties
Biofilms are usually found on solid substrates submerged in or exposed to an aqueous solution, although they can form as floating mats on liquid surfaces and also on the surface of leaves, particularly in high humidity climates. Given sufficient resources for growth, a biofilm will quickly grow to be macroscopic (visible to the naked eye). Biofilms can contain many different types of microorganism, e.g. bacteria, archaea, protozoa, fungi and algae; each group performs specialized metabolic functions. However, some organisms will form single-species films under certain conditions. The social structure (cooperation/competition) within a biofilm depends highly on the different species present.[22]
Extracellular matrix
The biofilm is held together and protected by a matrix of secreted polymeric compounds called EPS. EPS is an abbreviation for either extracellular polymeric substance or exopolysaccharide, although the latter one only refers to the polysaccharide moiety of EPS. In fact, the EPS matrix consists not only of polysaccharides but also of proteins (which may be the major component in environmental and waste water biofilms) and nucleic acids. A large proportion of the EPS is more or less strongly hydrated, however, hydrophobic EPS also occur; one example is cellulose[23] which is produced by a range of microorganisms. This matrix encases the cells within it and facilitates communication among them through biochemical signals as well as gene exchange. The EPS matrix is an important key to the evolutionary success of biofilms. One reason is that it traps extracellular enzymes and keeps them in close proximity to the cells. Thus, the matrix represents an external digestion system and allows for stable synergistic microconsortia of different species (Wingender and Flemming, Nat. Rev. Microbiol. 8, 623-633). Some biofilms have been found to contain water channels that help distribute nutrients and signalling molecules.[24] This matrix is strong enough that under certain conditions, biofilms can become fossilized (Stromatolites).
Bacteria living in a biofilm usually have significantly different properties from free-floating bacteria of the same species, as the dense and protected environment of the film allows them to cooperate and interact in various ways. One benefit of this environment is increased resistance to detergents and antibiotics, as the dense extracellular matrix and the outer layer of cells protect the interior of the community. In some cases antibiotic resistance can be increased a thousandfold.[25] Lateral gene transfer is greatly facilitated in biofilms and leads to a more stable biofilm structure.[26] Extracellular DNA is a major structural component of many different microbial biofilms.[27] Enzymatic degradation of extracellular DNA can weaken the biofilm structure and release microbial cells from the surface.
However, biofilms are not always less susceptible to antibiotics. For instance, the biofilm form of Pseudomonas aeruginosa has no greater resistance to antimicrobials than do stationary-phase planktonic cells, although when the biofilm is compared to logarithmic-phase planktonic cells, the biofilm does have greater resistance to antimicrobials. This resistance to antibiotics in both stationary-phase cells and biofilms may be due to the presence of persister cells.[28]
Habitat

Biofilms are ubiquitous. Nearly every species of microorganism, not only bacteria and archaea, have mechanisms by which they can adhere to surfaces and to each other. Biofilms will form on virtually every non-shedding surface in a non-sterile aqueous (or very humid) environment.
- Biofilms can be found on rocks and pebbles at the bottom of most streams or rivers and often form on the surface of stagnant pools of water. In fact, biofilms are important components of food chains in rivers and streams and are grazed by the aquatic invertebrates upon which many fish feed.
- Biofilms can grow in the most extreme environments: from, for example, the extremely hot, briny waters of hot springs ranging from very acidic to very alkaline, to frozen glaciers.
- In the human environment, biofilms can grow in showers very easily since they provide a moist and warm environment for the biofilm to thrive. Biofilms can form inside water and sewage pipes and cause clogging and corrosion. Biofilms on floors and counters can make sanitation difficult in food preparation areas.
- Biofilms in cooling- or heating-water systems are known to reduce heat transfer.[29]
- Biofilms in marine engineering systems, such as pipelines of the offshore oil and gas industry,[30] can lead to substantial corrosion problems. Corrosion is mainly due to abiotic factors; however, at least 20% of corrosion is caused by microorganisms that are attached to the metal subsurface (i.e., microbially influenced corrosion).
- Bacterial adhesion to boat hulls serves as the foundation for biofouling of seagoing vessels. Once a film of bacteria forms, it is easier for other marine organisms such as barnacles to attach. Such fouling can reduce maximum vessel speed by up to 20%, prolonging voyages and consuming fuel. Time in dry dock for refitting and repainting reduces the productivity of shipping assets, and the useful life of ships is also reduced due to corrosion and mechanical removal (scraping) of marine organisms from ships' hulls.
- Biofilms can also be harnessed for constructive purposes. For example, many sewage treatment plants include a secondary treatment stage in which waste water passes over biofilms grown on filters, which extract and digest organic compounds. In such biofilms, bacteria are mainly responsible for removal of organic matter (BOD), while protozoa and rotifers are mainly responsible for removal of suspended solids (SS), including pathogens and other microorganisms. Slow sand filters rely on biofilm development in the same way to filter surface water from lake, spring or river sources for drinking purposes. What we regard as clean water is effectively a waste material to these microcellular organisms.
- Biofilms can help eliminate petroleum oil from contaminated oceans or marine systems. The oil is eliminated by the hydrocarbon-degrading activities of microbial communities, in particular by a remarkable recently discovered group of specialists, the so-called hydrocarbonoclastic bacteria (HCB).[31]
- Stromatolites are layered accretionary structures formed in shallow water by the trapping, binding and cementation of sedimentary grains by microbial biofilms, especially of cyanobacteria. Stromatolites include some of the most ancient records of life on Earth, and are still forming today.
- Biofilms are present on the teeth of most animals as dental plaque, where they may cause tooth decay and gum disease.
- Biofilms are found on the surface of and inside plants. They can either contribute to crop disease or, as in the case of nitrogen-fixing Rhizobium on roots, exist symbiotically with the plant.[32] Examples of crop diseases related to biofilms include Citrus Canker, Pierce's Disease of grapes, and Bacterial Spot of plants such as peppers and tomatoes.[33]
- Biofilms are used in microbial fuel cells (MFCs) to generate electricity from a variety of starting materials, including complex organic waste and renewable biomass.[3][34][35]
- Recent studies in 2003 discovered that the immune system supports bio-film development in the large intestine. This was supported mainly with the fact that the two most abundantly produced molecules by the immune system also support bio-film production and are associated with the bio-films developed in the gut. This is especially important because the appendix holds a mass amount of these bacterial bio-films.[36] This discovery helps to distinguish the possible function of the appendix and the idea that the appendix can help reinoculate the gut with good gut flora.
Taxonomic diversity
Many different bacteria form biofilms, including gram-positive (e.g. Bacillus spp, Listeria monocytogenes, Staphylococcus spp, and lactic acid bacteria, including Lactobacillus plantarum and Lactococcus lactis) and gram-negative species (e.g. Escherichia coli, or Pseudomonas aeruginosa).[37]
Biofilms are formed by bacteria that colonize plants, e.g. Pseudomonas putida, Pseudomonas fluorescens, and related pseudomonads which are common plant-associated bacteria found on leaves, roots, and in the soil, and the majority of their natural isolates form biofilms.[38] Several nitrogen-fixing symbionts of legumes such as Rhizobium leguminosarum and Sinorhizobium meliloti form biofilms on legume roots and other inert surfaces.[38]
For other species in disease-associated biofilms see below.
Biofilms and infectious diseases
Biofilms have been found to be involved in a wide variety of microbial infections in the body, by one estimate 80% of all infections.[39] Infectious processes in which biofilms have been implicated include common problems such as bacterial vaginosis, urinary tract infections, catheter infections, middle-ear infections, formation of dental plaque,[40] gingivitis, coating contact lenses,[41] and less common but more lethal processes such as endocarditis, infections in cystic fibrosis, and infections of permanent indwelling devices such as joint prostheses and heart valves.[42][43] More recently it has been noted that bacterial biofilms may impair cutaneous wound healing and reduce topical antibacterial efficiency in healing or treating infected skin wounds.[44] Early detection of biofilms in wounds is crucial to successful chronic wound management. Although many techniques have developed to identify planktonic bacteria in viable wounds, few have been able to quickly and accurately identify bacterial biofilms. Future studies are needed to find means of identifying and monitoring biofilm colonization at the bedside to permit timely initiation of treatment.[45]
It has recently been shown that biofilms are present on the removed tissue of 80% of patients undergoing surgery for chronic sinusitis. The patients with biofilms were shown to have been denuded of cilia and goblet cells, unlike the controls without biofilms who had normal cilia and goblet cell morphology.[46] Biofilms were also found on samples from two of 10 healthy controls mentioned. The species of bacteria from interoperative cultures did not correspond to the bacteria species in the biofilm on the respective patient's tissue. In other words, the cultures were negative though the bacteria were present.[47]
Biofilms can also be formed on the inert surfaces of implanted devices such as catheters, prosthetic cardiac valves and intrauterine devices. [48]
New staining techniques are being developed to differentiate bacterial cells growing in living animals, e.g. from tissues with allergy-inflammations.[49]
Research has shown that sub-therapeutic levels of β-lactam antibiotics induce biofilm formation in Staphylococcus aureus. This sub-therapeutic level of antibiotic may result from the use of antibiotics as growth promoters in agriculture, or during the normal course of antibiotic therapy. The biofilm formation induced by low-level methicillin was inhibited by DNase, suggesting that the sub-therapeutic levels of antibiotic also induce extracellular DNA release.[50] Moreover, from an evolutionary point of view, the creation of the tragedy of the commons in pathogenic microbes may provide advanced therapeutic ways for chronic infections caused by biofilms via genetically engineered invasive cheaters who can invade wild-types ‘cooperators’ of pathogenic bacteria until cooperator populations go to extinction or overall population ‘cooperators and cheaters ’ go to extinction.[51]
Dental plaque
Dental plaque is an oral biofilm that adheres to the teeth and consists of many species of both bacteria and fungi (such as Streptococcus mutans and Candida albicans), embedded in salivary polymers and microbial extracellular products. The accumulation of microorganisms subjects the teeth and gingival tissues to high concentrations of bacterial metabolites which results in dental disease.[52]
The biofilm on the surface of teeth is frequently subject to oxidative stress[53] and acid stress.[54] Dietary carbohydrates can cause a dramatic decrease in pH in oral biofilms to values of 4 and below (acid stress).[54] A pH of 4 at body temperature of 37 °C causes depurination of DNA, leaving apurinic (AP) sites in DNA,[55] especially loss of guanine.[56]
The Dental plaque biofilm can result in the disease dental caries if it is allowed to develop over time. An ecologic shift away from balanced populations within the dental biofilm is driven by certain (cariogenic) microbiological populations beginning to dominate when the environment favours them. The shift to an Acidogenic, aciduric, and cariogenic microbiological population develops and is maintained by frequent consumption of fermentable dietary carbohydrate. The resulting activity shift in the biofilm (and resulting acid production within the biofilm, at the tooth surface) is associated with an imbalance between demineralization and remineralisation leading to net mineral loss within dental hard tissues (enamel and then dentin), the sign and symptom being a carious lesion. By preventing the Dental plaque biofilm from maturing or by returning it back to a non-cariogenic state, dental caries can be prevented and arrested.[57] This can be achieved though the behavioural step of reducing the supply of fermentable carbohydrates (i.e. sugar intake) and frequent removal of the biofilm (i.e. toothbrushing).
A peptide pheromone quorum sensing signaling system in S. mutans includes the Competence Stimulating Peptide (CSP) that controls genetic competence.[58][59] Genetic competence is the ability of a cell to take up DNA released by another cell. Competence can lead to genetic transformation, a form of sexual interaction, favored under conditions of high cell density and/or stress where there is maximal opportunity for interaction between the competent cell and the DNA released from nearby donor cells. This system is optimally expressed when S. mutans cells reside in an actively growing biofilm. Biofilm grown S. mutans cells are genetically transformed at a rate 10- to 600-fold higher than S. mutans growing as free-floating planktonic cells suspended in liquid.[58]
When the biofilm, containing S. mutans and related oral streptococci, is subjected to acid stress, the competence regulon is induced, leading to resistance to being killed by acid.[54] As pointed out by Michod et al., transformation in bacterial pathogens likely provides for effective and efficient recombinational repair of DNA damages.[60] It appears that S. mutans can survive the frequent acid stress in oral biofilms, in part, through the recombinational repair provided by competence and transformation.
Streptococcus pneumoniae
S. pneumoniae is the main cause of community-acquired pneumonia and meningitis in children and the elderly, and of septicemia in HIV-infected persons. When S. pneumonia grows in biofilms, genes are specifically expressed that respond to oxidative stress and induce competence.[61] Formation of a biofilm depends on competence stimulating peptide (CSP). CSP also functions as a quorum-sensing peptide. It not only induces biofilm formation, but also increases virulence in pneumonia and meningitis.
It has been proposed that competence development and biofilm formation is an adaptation of S. pneumoniae to survive the defenses of the host.[60] In particular, the host’s polymorphonuclear leukocytes produce an oxidative burst to defend against the invading bacteria, and this response can kill bacteria by damaging their DNA. Competent S. pneumoniae in a biofilm have the survival advantage that they can more easily take up transforming DNA from nearby cells in the biofilm to use for recombinational repair of oxidative damages in their DNA. Competent S. pneumoniae can also secrete an enzyme (murein hydrolase) that destroys non-competent cells (fratricide) causing DNA to be released into the surrounding medium for potential use by the competent cells.[62]
Biofilms in medicine
The rapidly expanding worldwide industry for biomedical devices and tissue engineering related products is already at $180 billion per year, yet this industry continues to suffer from microbial colonization. No matter the sophistication, microbial infections can develop on all medical devices and tissue engineering constructs.[63]
60-70% of nosocomial or hospital acquired infections are associated with the implantation of a biomedical device.[63] This leads to 2 million cases annually in the U.S., costing the healthcare system over $5 billion in additional healthcare expenses.[63]
If an infection develops a biofilm, it becomes even harder to treat. As the bacteria changes, it becomes more resistant to antibiotics and the body's own host defenses.[63]
Biofilms in the food industry
Biofilms have become problematic in several food industries due to the ability to form on plants and during industrial processes.[64] Bacteria can survive long periods of time in water, animal manure, and soil, causing biofilm formation on plants or in the processing equipment.[65] The buildup of biofilms can affect the heat flow across a surface and increase surface corrosion and frictional resistance of fluids.[66] These can lead to a loss of energy in a system and overall loss of products.[66] Along with economic problems biofilm formation on food poses a health risk to consumers due to the ability to make the food more resistant to disinfectants [64] As a result, from 1996 to 2010 the Center for Disease Control and Prevention estimated 48 million foodborne illnesses per year.[64] Biofilms have been connected to about 80% of bacterial infections in the United States.[64]
In produce, microorganisms attach to the surfaces and biofilms develop internally.[64] During the washing process, biofilms resist sanitization and allow bacteria to spread across the produce.[64] This problem is also found in ready to eat foods because the foods go through limited cleaning procedures before consumption [64] Due to the perishability of dairy products and limitations in cleaning procedures, resulting in the buildup of bacteria, dairy is susceptible to biofilm formation and contamination.[64][66] The bacteria can spoil the products more readily and contaminated products pose a health risk to consumers. One bacteria that can be found in various industries and is a major cause of foodborne disease is Salmonella.[67] Large amounts of salmonella contamination can be found in the poultry processing industry as about 50% of salmonella strains can produce biofilms on poultry farms.[64] Salmonella increases the risk of foodborne illnesses when the poultry products are not cleaned and cooked correctly. Salmonella is also found in the seafood industry where biofilms form from seafood borne pathogens on the seafood itself as well as in water.[67] Shrimp products are commonly affected by salmonella because of unhygienic processing and handling techniques[67] The preparation practices of shrimp and other seafood products can allow for bacteria buildup on the products.[67]
New forms of cleaning procedures are being tested in order to reduce biofilm formation in these processes which will lead to safer and more productive food processing industries. These new forms of cleaning procedures also have a profound effect on the environment, often releasing toxic gases into the groundwater reservoirs.[66]
See also
- Bacterial nanowires
- Center for Biofilm Engineering
- Chemistry of biofilm prevention
- Kombucha
- Microbial mat
- Phage therapy
- Phototrophic biofilms
- Stromatolite
- List of bacterial vaginosis microbiota
References
- ↑ Vert, Michel; Doi, Yoshiharu; Hellwich, Karl-Heinz; Hess, Michael; Hodge, Philip; Kubisa, Przemyslaw; Rinaudo, Marguerite; Schué, François (2012). "Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)" (PDF). Pure and Applied Chemistry. 84 (2): 377–410. doi:10.1351/PAC-REC-10-12-04.
- ↑ Hall-Stoodley L, Costerton JW, Stoodley P (February 2004). "Bacterial biofilms: from the natural environment to infectious diseases". Nature Reviews Microbiology. 2 (2): 95–108. doi:10.1038/nrmicro821. PMID 15040259.
- 1 2 Lear G, Lewis GD, eds. (2012). Microbial Biofilms: Current Research and Applications. Caister Academic Press. ISBN 978-1-904455-96-7.
- ↑ Karatan E, Watnick P (June 2009). "Signals, regulatory networks, and materials that build and break bacterial biofilms". Microbiology and Molecular Biology Reviews. 73 (2): 310–47. doi:10.1128/MMBR.00041-08. PMC 2698413
 . PMID 19487730.
. PMID 19487730. - ↑ Hoffman LR, D'Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI (August 2005). "Aminoglycoside antibiotics induce bacterial biofilm formation". Nature. 436 (7054): 1171–5. doi:10.1038/nature03912. PMID 16121184. (primary source)
- ↑ An D, Parsek MR (June 2007). "The promise and peril of transcriptional profiling in biofilm communities". Current Opinion in Microbiology. 10 (3): 292–6. doi:10.1016/j.mib.2007.05.011. PMID 17573234.
- ↑ Briandet, R., Herry, J.M., Bellon-Fontaine, M.N., 2001. "Determination of the van der Waals, electron donor and electron acceptor surface tension components of static Gram-positive microbial biofilms." Colloids and Surfaces B: Biointerfaces, Vol. 21, Issue 4, pg 299-310.
- ↑ Takahashi, H., Suda, T., Tanaka, Y., Kimura, B., 2010. "Cellular hydrophobicity of Listeria monocytogenes involves initial attachment and biofilm formation on the surface of polyvinyl chloride." Applied Microbiology, Vol. 50, Iss. 6, pgs 618-625.
- 1 2 3 Donlan, Rodney M. 2002. Biofilms: Microbial Life on Surfaces. Emerging Infectious Diseases. Vol. 8, No. 9: pg. 881-890.
- ↑ Quorum-Sensing Regulation of the Biofilm Matrix Genes (pel) of Pseudomonas aeruginosa
- ↑ Monroe, Don (2007). "Looking for Chinks in the Armor of Bacterial Biofilms". PLoS Biology. 5 (11): e307. doi:10.1371/journal.pbio.0050307. ISSN 1545-7885.
- ↑ Kaplan JB, Ragunath C, Ramasubbu N, Fine DH (August 2003). "Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta-hexosaminidase activity". Journal of Bacteriology. 185 (16): 4693–8. doi:10.1128/JB.185.16.4693-4698.2003. PMC 166467
 . PMID 12896987.
. PMID 12896987. - ↑ Izano EA, Amarante MA, Kher WB, Kaplan JB (January 2008). "Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms". Applied and Environmental Microbiology. 74 (2): 470–6. doi:10.1128/AEM.02073-07. PMC 2223269
 . PMID 18039822.
. PMID 18039822. - ↑ Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N (July 2004). "Enzymatic detachment of Staphylococcus epidermidis biofilms". Antimicrobial Agents and Chemotherapy. 48 (7): 2633–6. doi:10.1128/AAC.48.7.2633-2636.2004. PMC 434209
 . PMID 15215120.
. PMID 15215120. - ↑ Xavier JB, Picioreanu C, Rani SA, van Loosdrecht MC, Stewart PS (December 2005). "Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix--a modelling study". Microbiology. 151 (Pt 12): 3817–32. doi:10.1099/mic.0.28165-0. PMID 16339929.
- ↑ Davies DG, Marques CN (March 2009). "A fatty acid messenger is responsible for inducing dispersion in microbial biofilms". Journal of Bacteriology. 191 (5): 1393–403. doi:10.1128/JB.01214-08. PMC 2648214
 . PMID 19074399.
. PMID 19074399. - ↑ Barraud N, Hassett DJ, Hwang SH, Rice SA, Kjelleberg S, Webb JS (2006). "Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa". Journal of Bacteriology. 188 (21): 7344–7353. doi:10.1128/jb.00779-06. PMC 1636254
 . PMID 17050922.
. PMID 17050922. - ↑ Barraud N, Storey MV, Moore ZP, Webb JS, Rice SA, Kjelleberg S (2009). "Nitric oxide-mediated dispersal in single- and multi-species biofilms of clinically and industrially relevant microorganisms". Microbial Biotechnology. 2 (3): 370–378. doi:10.1111/j.1751-7915.2009.00098.x. PMC 3815757
 . PMID 21261931.
. PMID 21261931. - ↑ "Dispersal of Biofilm in Cystic Fibrosis using Low Dose Nitric Oxide". University of Southampton. Retrieved 20 January 2012.
- 1 2 Chua SL, Liu Y, Yam JK, Tolker-Nielsen T, Kjelleberg S, Givskov M, Yang L (2014). "Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles". Nature Communications. 5. doi:10.1038/ncomms5462.
- ↑ S L Chua; L D Hultqvist; M Yuan; M Rybtke; T E Nielsen; M Givskov; T Tolker-Nielsen; L Yang (2015). "In vitro and in vivo generation and characterization of Pseudomonas aeruginosa biofilm–dispersed cells via c-di-GMP manipulation". Nature Protcols. 10 (8): 1165–1180. doi:10.1038/nprot.2015.067.
- ↑ Nadell, Carey D.; Xavier, Joao B.; Foster, Kevin R. (1 January 2009). "The sociobiology of biofilms". FEMS Microbiology Reviews. 33 (1): 206–224. doi:10.1111/j.1574-6976.2008.00150.x. PMID 19067751.
- ↑ Choong, Ferdinand X; Bäck, Marcus; Fahlén, Sara; Johansson, Leif BG; Melican, Keira; Rhen, Mikael; Nilsson, K Peter R; Richter-Dahlfors, Agneta (23 November 2016). "Real-time optotracing of curli and cellulose in live Salmonella biofilms using luminescent oligothiophenes". npj Biofilms and Microbiomes (2): 16024. doi:10.1038/npjbiofilms.2016.24. Retrieved 24 November 2016.
- ↑ Stoodley, Paul; Dirk deBeer; Zbigniew Lewandowski (August 1994). "Liquid Flow in Biofilm Systems". Appl Environ Microbiol. 60 (8): 2711–2716. PMID 16349345.
- ↑ Stewart PS, Costerton JW (July 2001). "Antibiotic resistance of bacteria in biofilms". Lancet. 358 (9276): 135–8. doi:10.1016/S0140-6736(01)05321-1. PMID 11463434.
- ↑ Molin S, Tolker-Nielsen T (June 2003). "Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure". Current Opinion in Biotechnology. 14 (3): 255–61. doi:10.1016/S0958-1669(03)00036-3. PMID 12849777.
- ↑ Jakubovics NS, Shields RC, Rajarajan N, Burgess JG (December 2013). "Life after death: the critical role of extracellular DNA in microbial biofilms". Lett. Appl. Microbiol. 57 (6): 467–75. doi:10.1111/lam.12134. PMID 23848166.
- ↑ Spoering AL, Lewis K (December 2001). "Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials". Journal of Bacteriology. 183 (23): 6746–51. doi:10.1128/JB.183.23.6746-6751.2001. PMC 95513
 . PMID 11698361.
. PMID 11698361. - ↑ Characklis, WG; Nevimons, MJ; Picologlou, BF (1981). "Influence of Fouling Biofilms on Heat Transfer". Heat Transfer Engineering. 3: 23–37. doi:10.1080/01457638108939572.
- ↑ Schwermer CU, Lavik G, Abed RM, et al. (May 2008). "Impact of nitrate on the structure and function of bacterial biofilm communities in pipelines used for injection of seawater into oil fields". Applied and Environmental Microbiology. 74 (9): 2841–51. doi:10.1128/AEM.02027-07. PMC 2394879
 . PMID 18344353.
. PMID 18344353. - ↑ Martins dos Santos VA, Yakimov MM, Timmis KN, Golyshin PN (2008). "Genomic Insights into Oil Biodegradation in Marine Systems". In Díaz E. Microbial Biodegradation: Genomics and Molecular Biology. Horizon Scientific Press. p. 1971. ISBN 978-1-904455-17-2.
- ↑ "Introduction to Biofilms: Desirable and undesirable impacts of biofilm". (primary source)
- ↑ Andersen PC, Brodbeck BV, Oden S, Shriner A, Leite B (September 2007). "Influence of xylem fluid chemistry on planktonic growth, biofilm formation and aggregation of Xylella fastidiosa". FEMS Microbiology Letters. 274 (2): 210–7. doi:10.1111/j.1574-6968.2007.00827.x. PMID 17610515.
- ↑ Chua SL, Wang VB, Cai Z, Sivakumar K, Kjelleberg S, Cao B, Loo SC, Yang L (2014). "A stable synergistic microbial consortium for simultaneous azo dye removal and bioelectricity generation". Bioresource technology. 155: 71–76. doi:10.1016/j.biortech.2013.12.078. PMID 24434696.
- ↑ Chua SL, Wang VB, Cao B, Loo SC, Yang L (2013). "A stable synergistic microbial consortium for simultaneous azo dye removal and bioelectricity generation". PLoS ONE. 8 (5): e63129. doi:10.1371/journal.pone.0063129. PMC 3659106
 . PMID 23700414.
. PMID 23700414. - ↑ Bollinger, Randal; Barbas, Andrew; Bush, Errol; Lin, Shu; Parker, William (24 June 2007). "Biofilms in the large bowel suggest an apparent function of the human vermiform appendix" (PDF). The Journal of Theoretical Biology. 249 (4): 826–831. doi:10.1016/j.jtbi.2007.08.032. PMID 17936308.
- ↑ Abee, T; Kovács, A. T.; Kuipers, O. P.; Van Der Veen, S (2011). "Biofilm formation and dispersal in Gram-positive bacteria". Current Opinion in Biotechnology. 22 (2): 172–9. doi:10.1016/j.copbio.2010.10.016. PMID 21109420.
- 1 2 Danhorn, T; Fuqua, C (2007). "Biofilm formation by plant-associated bacteria". Annual Review of Microbiology. 61: 401–22. doi:10.1146/annurev.micro.61.080706.093316. PMID 17506679.
- ↑ "Research on microbial biofilms (PA-03-047)". NIH, National Heart, Lung, and Blood Institute. 2002-12-20.
- ↑ Rogers, Anthony (2008). Molecular Oral Microbiology. Caister Academic Press. pp. 88–91. ISBN 978-1-904455-24-0.
- ↑ Imamura Y, Chandra J, Mukherjee PK, et al. (January 2008). "Fusarium and Candida albicans biofilms on soft contact lenses: model development, influence of lens type, and susceptibility to lens care solutions". Antimicrobial Agents and Chemotherapy. 52 (1): 171–82. doi:10.1128/AAC.00387-07. PMC 2223913
 . PMID 17999966.
. PMID 17999966. - ↑ Lewis K (April 2001). "Riddle of biofilm resistance". Antimicrobial Agents and Chemotherapy. 45 (4): 999–1007. doi:10.1128/AAC.45.4.999-1007.2001. PMC 90417
 . PMID 11257008.
. PMID 11257008. - ↑ Parsek MR, Singh PK (2003). "Bacterial biofilms: an emerging link to disease pathogenesis". Annual Review of Microbiology. 57: 677–701. doi:10.1146/annurev.micro.57.030502.090720. PMID 14527295.
- ↑ Davis SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM (2008). "Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo". Wound Repair and Regeneration. 16 (1): 23–9. doi:10.1111/j.1524-475X.2007.00303.x. PMID 18211576.
- ↑ Vyas KS, Wong LK (2016). "Detection of Biofilm in Wounds as an Early Indicator for Risk for Tissue Infection and Wound Chronicity". Ann Plast Surg. 76 (1): 127–31. doi:10.1097/SAP.0000000000000440. PMID 25774966.
- ↑ Sanclement J, Webster P, Thomas J, Ramadan H (2005). "Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis". Laryngoscope. 115 (4): 578–82. doi:10.1097/01.mlg.0000161346.30752.18. PMID 15805862.
- ↑ Sanderson AR, Leid JG, Hunsaker D (July 2006). "Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis". The Laryngoscope. 116 (7): 1121–6. doi:10.1097/01.mlg.0000221954.05467.54. PMID 16826045.
- ↑ Auler ME, Morreira D, Rodrigues FF, et al. (April 2009). "Biofilm formation on intrauterine devices in patients with recurrent vulvovaginal candidiasis". Medical Mycology. 48 (1): 211–6. doi:10.3109/13693780902856626. PMID 20055746.
- ↑ Leevy WM, Gammon ST, Jiang H, et al. (December 2006). "Optical imaging of bacterial infection in living mice using a fluorescent near-infrared molecular probe". Journal of the American Chemical Society. 128 (51): 16476–7. doi:10.1021/ja0665592. PMC 2531239
 . PMID 17177377.
. PMID 17177377. - ↑ Kaplan JB, Izano EA, Gopal P, et al. (2012). "Low Levels of β-Lactam Antibiotics Induce Extracellular DNA Release and Biofilm Formation in Staphylococcus aureus". MBio. 3 (4): e00198–12. doi:10.1128/mBio.00198-12. PMC 3419523
 . PMID 22851659.
. PMID 22851659. - ↑ Ibrahim, Ahmed (2015): The tragedy of the commons and prisoner's dilemma may improve our realization of the theory of life and provide us with advanced therapeutic ways. figshare.
- ↑ Augustin Mihai; Carmen Balotescu-Chifiriuc; Veronica Lazăr; Ruxandra Stănescu; Mihai Burlibașa; Dana Catrinel Ispas (Dec 2010). "Microbial biofilms in dental medicine in reference to implanto-prostethic rehabilitation". Revista de chirurgie oro-maxilo-facială și implantologie (in Romanian). 1 (1): 9–13. ISSN 2069-3850. 8. Retrieved 2012-06-03.(webpage has a translation button)
- ↑ Marquis RE (September 1995). "Oxygen metabolism, oxidative stress and acid-base physiology of dental plaque biofilms". J. Ind. Microbiol. 15 (3): 198–207. doi:10.1007/bf01569826. PMID 8519478.
- 1 2 3 Lemos JA, Abranches J, Burne RA (January 2005). "Responses of cariogenic streptococci to environmental stresses" (PDF). Curr Issues Mol Biol. 7 (1): 95–107. PMID 15580782.
- ↑ TAMM C, HODES ME, CHARGAFF E (March 1952). "The formation apurinic acid from the desoxyribonucleic acid of calf thymus". J. Biol. Chem. 195 (1): 49–63. PMID 14938354.
- ↑ FREESE EB (April 1961). "Transitions and transversions induced by depurinating agents". Proc. Natl. Acad. Sci. U.S.A. 47 (4): 540–5. doi:10.1073/pnas.47.4.540. PMC 221484
 . PMID 13701660.
. PMID 13701660. - ↑ Fejerskov, Ole (2015). athology of dental caries. In: Dental caries: the disease and its clinical management. Oxford (UK): Wiley Blackwell. pp. 7–9. ISBN 978-1405138895.
- 1 2 Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG (February 2001). "Natural genetic transformation of Streptococcus mutans growing in biofilms". J. Bacteriol. 183 (3): 897–908. doi:10.1128/JB.183.3.897-908.2001. PMC 94956
 . PMID 11208787.
. PMID 11208787. - ↑ Senadheera D, Cvitkovitch DG (2008). "Quorum sensing and biofilm formation by Streptococcus mutans". Adv. Exp. Med. Biol. Advances in Experimental Medicine and Biology. 631: 178–88. doi:10.1007/978-0-387-78885-2_12. ISBN 978-0-387-78884-5. PMID 18792689.
- 1 2 Michod RE, Bernstein H, Nedelcu AM (May 2008). "Adaptive value of sex in microbial pathogens". Infect. Genet. Evol. 8 (3): 267–85. doi:10.1016/j.meegid.2008.01.002. PMID 18295550.http://www.hummingbirds.arizona.edu/Faculty/Michod/Downloads/IGE%20review%20sex.pdf
- ↑ Oggioni MR, Trappetti C, Kadioglu A, Cassone M, Iannelli F, Ricci S, Andrew PW, Pozzi G (September 2006). "Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis". Mol. Microbiol. 61 (5): 1196–210. doi:10.1111/j.1365-2958.2006.05310.x. PMC 1618759
 . PMID 16925554.
. PMID 16925554. - ↑ Wei H, Håvarstein LS (August 2012). "Fratricide is essential for efficient gene transfer between pneumococci in biofilms". Appl. Environ. Microbiol. 78 (16): 5897–905. doi:10.1128/AEM.01343-12. PMC 3406168
 . PMID 22706053.
. PMID 22706053. - 1 2 3 4 Bryers J. D. (2008). "Medical biofilms". Biotechnology and Bioengineering. 100 (1): 1–18. doi:10.1002/bit.21838. PMC 2706312
 . PMID 18366134.
. PMID 18366134. - 1 2 3 4 5 6 7 8 9 Srey S. "Biofilm formation in food industries: A food safety concern".
- ↑ T. Tarver, "Biofilms: A Threat to Food Safety – IFT.org", Ift.org, 2016.
- 1 2 3 4 Kumar C. "Significance of microbial biofilms in food industry: a review".
- 1 2 3 4 Mizan F. "Microbial biofilms in seafood: A food-hygiene challenge".
Further reading
- Ramadan HH, Sanclement JA, Thomas JG (March 2005). "Chronic rhinosinusitis and biofilms". Otolaryngology-Head and Neck Surgery. 132 (3): 414–7. doi:10.1016/j.otohns.2004.11.011. PMID 15746854.
- Bendouah Z, Barbeau J, Hamad WA, Desrosiers M (June 2006). "Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis". Otolaryngology-Head and Neck Surgery. 134 (6): 991–6. doi:10.1016/j.otohns.2006.03.001. PMID 16730544.
- Lynch AS, Robertson GT (2008). "Bacterial and fungal biofilm infections". Annual Review of Medicine. 59: 415–28. doi:10.1146/annurev.med.59.110106.132000. PMID 17937586.
- Vo P, Nunez M (2010). "Bdellovibrio bacteriovorus Predation in Dual-Species Biofilms of E. coli Prey and M. luteus Decoys". arXiv:1005.3582
 [q-bio.PE].
[q-bio.PE]. - Allison, D. G. (2000). Community structure and co-operation in biofilms. Cambridge, UK: Cambridge University Press. ISBN 0-521-79302-5.
- Lynch, James F.; Lappin-Scott, Hilary M.; Costerton, J. W. (2003). Microbial biofilms. Cambridge, UK: Cambridge University Press. ISBN 0-521-54212-X.
- Fratamico, M. (2009). Biofilms in the food and beverage industries. Woodhead Publishing Limited. ISBN 978-1-84569-477-7.
- Chua SL, Liu Y, Yam JK, Tolker-Nielsen T, Kjelleberg S, Givskov M, Yang L (2014). "Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles". Nature Communications. 5. doi:10.1038/ncomms5462.
- Chua SL, Tolker-Nielsen T, Kjelleberg S, Givskov M, Yang L (2013). "Bis-(3′-5′)-cyclic dimeric GMP regulates antimicrobial peptide resistance in Pseudomonas aeruginosa". Antimicrobial Agents and Chemotherapy. 57 (5): 2066–2075. doi:10.1128/AAC.02499-12. PMC 3632963
 . PMID 23403434.
. PMID 23403434.
External links
- Thickness analysis, organic and mineral proportion of biofilms in order to decide a treatment strategy
- Biofilm Archive of Biofilm Research & News
- "Why Am I Still Sick?" - The Movie, 2012: Documentary on Biofilms: The Silent Role of Biofilms in Chronic Disease
- HD Video Interviews on biofilms, antibiotics, etc. with experts, youtube.com: ADRSupport/biofilm