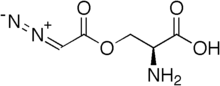
Azaserine
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| |
| CAS Number |
115-02-6 |
| PubChem (CID) | 5284344 |
| ChemSpider |
16735688 |
| UNII |
87299V3Q9W |
| KEGG |
D03032 |
| ChEBI |
CHEBI:74846 |
| ChEMBL |
CHEMBL1095699 |
| Chemical and physical data | |
| Formula | C5H7N3O4 |
| Molar mass | 173.127 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Azaserine is a naturally occurring serine derivative diazo compound with antineoplastic and antibiotic properties deriving from its action as a purinergic antagonist and structural similarity to glutamine. Azazerine acts by competitively inhibiting glutamine amidotransferase, a key enzyme responsible for glutamine metabolism.
Mechanism of Action
Azaserine inhibits the rate limiting step of the metabolic hexosamine pathway and an irreversibly inhibits γ-glutamyltransferase by acting directly at the substrate-binding pocket. Independent of hexosamine pathway inhibition, azaserine has been demonstrated to protect against hyperglycemic endothelial damage by elevating serum concentrations of manganese-superoxide dismutase, directly reducing the concentration of reactive oxygen species.
Azaserine also downregulates the expression of VCAM-1 and ICAM-1 in response to TNF-α, and research indicates that it may have potential in identifying the L-leucine-favoring system transporter in human T-lymphocytes.
Properties
Azaserine has a solubility of 50 mg/mL in water, a melting point of 146-162 °C, a vapor pressure of 1.53x10−10mmHg at 25 °C, and decomposes before melting.
References
- Segel, G.B., et al. 1989. J. Biol. Chem. 264: 16399-16402. PMID 2789219
- Hull, R.L., et al. 2007. Am. J. Physiol., Cell Physiol. 293: C1586-C1593. PMID 17804609
- Wada, K., et al. 2008. J. Mol. Biol. 380: 361-372. PMID 18555071
- Rajapakse, A.G., et al. 2009. Am. J. Physiol. Heart Circ. Physiol. 296: H815-H822. PMID 19136606
- Angana Gupta Rajapakse, Xiu-Fen Ming, João M. Carvas, and Zhihong Yang. The hexosamine biosynthesis inhibitor azaserine prevents endothelial inflammation and dysfunction under hyperglycemic condition through antioxidant effects. http://ajpheart.physiology.org/content/296/3/H815.full
- Lebedeva ZI, Kabanova EA, Berezov TT. 1986 Mar;12(3):413-20. 6-diazo-5-oxo-L-norleucine and azaserine as affinity inhibitors of glutamin(asparagin)ase.