Amylocaine
 | |
| Names | |
|---|---|
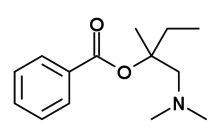
| IUPAC name
benzoic acid [1-(dimethylaminomethyl)-1-methylpropyl] ester | |
| Identifiers | |
| 644-26-8 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1740065 |
| ChemSpider | 10312 |
| ECHA InfoCard | 100.010.375 |
| KEGG | D07454 |
| PubChem | 10767 |
| UNII | QRW683O56T |
| |
| |
| Properties | |
| C14H21NO2 | |
| Molar mass | 235.32204 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Amylocaine was the first synthetic local anesthetic. It was synthesized and patented under the name Stovaine by Ernest Fourneau at the Pasteur Institute in 1903.[1] It used to be used mostly in spinal anesthesia.[2]
Notes and references
- ↑ Fourneau, E. (1904). "Stovaïne, anesthésique local". Bulletin des sciences pharmacologiques. 10: 141-148.
- ↑ Debue-Barazer, Christine (2007). "Les Implications scientifiques et industrielles du succès de la Stovaïne : Ernest Fourneau (1872-1949) et la chimie des médicaments en France". Gesnerus 64 (1-2): 24-53.
See also
- Dimethylaminopivalophenone, an opioid with a similar SAR (an amine that is a sole methylene spacer shorter)
External links
- Smith, Maurice I.; Hatcher, Robert A. (January 1917). "A Contribution to the Pharmacology of Stovaine". Journal of Pharmacology and Experimental Therapeutics. 9 (4): 231–240.
- Ball, Christine M.; Westhorpe, Rod N. (2004). "Local Anaesthesia after Cocaine". Anaesthesia and Intensive Care. 32 (2): 157. PMID 15957711.
This article is issued from Wikipedia - version of the 5/10/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.