Aluminium nitride
 | |
 | |
| Names | |
|---|---|
| Other names
Aluminium nitride | |
| Identifiers | |
| 24304-00-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:50884 |
| ChemSpider | 81668 |
| ECHA InfoCard | 100.041.931 |
| EC Number | 246-140-8 |
| PubChem | 90455 |
| RTECS number | BD1055000 |
| |
| |
| Properties | |
| AlN | |
| Molar mass | 40.9882 g/mol |
| Appearance | white to pale-yellow solid |
| Density | 3.260 g/cm3 |
| Melting point | 2,200 °C (3,990 °F; 2,470 K) |
| Boiling point | 2,517 °C (4,563 °F; 2,790 K) decomposes |
| hydrolyses (powder), insoluble (monocrystalline) | |
| Solubility | insoluble, subject of hydrolysis in water solutions of bases and acids [2] |
| Band gap | 6.015 eV [3] (direct) |
| Electron mobility | ~300 cm2/(V·s) |
| Thermal conductivity | 285 W/(m·K) |
| Refractive index (nD) |
1.9–2.2 |
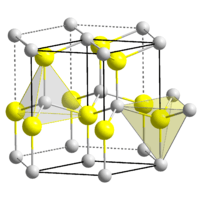
| Structure | |
| Wurtzite | |
| C6v4-P63mc | |
| Tetrahedral | |
| Thermochemistry | |
| 30.1 J/mol K | |
| Std molar entropy (S |
20.2 J/mol K |
| Std enthalpy of formation (ΔfH |
318 kJ/mol |
| Gibbs free energy (ΔfG˚) |
287.4 kJ/mol |
| Hazards | |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Aluminium nitride (AlN) is a nitride of aluminium. Its wurtzite phase (w-AlN) is a wide band gap (6.01-6.05 eV at room temperature) semiconductor material, giving it potential application for deep ultraviolet optoelectronics.
History
AlN was first synthesized in 1877, but it was not until the middle of the 1980s that its potential for application in microelectronics was realized due to its relatively high thermal conductivity for an electrically insulating ceramic (70–210 W·m−1·K−1 for polycrystalline material, and as high as 285 W·m−1·K−1 for single crystals).[4]
Stability and chemical properties
Aluminium nitride is stable at high temperatures in inert atmospheres and melts at 2800 °C. In a vacuum, AlN decomposes at ~1800 °C. In the air, surface oxidation occurs above 700 °C, and even at room temperature, surface oxide layers of 5-10 nm have been detected. This oxide layer protects the material up to 1370 °C. Above this temperature bulk oxidation occurs. Aluminium nitride is stable in hydrogen and carbon dioxide atmospheres up to 980 °C.[5]
The material dissolves slowly in mineral acids through grain boundary attack, and in strong alkalies through attack on the aluminium nitride grains. The material hydrolyzes slowly in water. Aluminium nitride is resistant to attack from most molten salts, including chlorides and cryolite.
Manufacture
AlN is synthesized by the carbothermal reduction of aluminium oxide in the presence of gaseous nitrogen or ammonia or by direct nitridation of aluminium. The use of sintering aids, such as Y2O3 or CaO, and hot pressing is required to produce a dense technical grade material.
Applications
Epitaxially grown thin film crystalline aluminium nitride is used for surface acoustic wave sensors (SAWs) deposited on silicon wafers because of AlN's piezoelectric properties. One application is an RF filter which is widely used in mobile phones,[6] which is called a thin film bulk acoustic resonator (FBAR). This is a MEMS device that uses aluminium nitride sandwiched between two metal layers.[7]
Aluminium nitride is also used to build piezoelectric micromachined ultrasound transducers, which emit and receive ultrasound and which can be used for in-air rangefinding over distances of up to a meter.[8][9]
Metallization methods are available to allow AlN to be used in electronics applications similar to those of alumina and beryllium oxide. AlN nanotubes as inorganic quasi-one-dimensional nanotubes, which are isoelectronic with carbon nanotubes, have been suggested as chemical sensors for toxic gases.[10][11]
Currently there is much research into developing light-emitting diodes to operate in the ultraviolet using gallium nitride based semiconductors and, using the alloy aluminium gallium nitride, wavelengths as short as 250 nm have been achieved. In May 2006, an inefficient AlN LED emission at 210 nm was reported.[12]
There are also multiple research efforts in industry and academia to use aluminum nitride in piezoelectric MEMS applications. These include resonators, gyroscopes and microphones.[13][14]
Among the applications of AlN are
- opto-electronics,
- dielectric layers in optical storage media,
- electronic substrates, chip carriers where high thermal conductivity is essential,
- military applications,
- as a crucible to grow crystals of gallium arsenide,
- steel and semiconductor manufacturing.
See also
References
- ↑ "Aluminium Nitride". Accuratus. Retrieved 2014-01-01.
- ↑ Fukumoto, S.; Hookabe, T.; Tsubakino, H. (2010). "Hydrolysis behavior of aluminum nitride in various solutions". J. Mat. Science. 35 (11): 2743–2748. doi:10.1023/A:1004718329003.
- ↑ Feneberg, M.; Leute, R. A. R.; Neuschl, B.; Thonke, K.; Bickermann, M. (2010). Phys. Rev. B. 82: 075208. doi:10.1103/physrevb.82.075208.
- ↑ "AlN - Aluminium Nitride". Ioffe Database. Sankt-Peterburg: FTI im. A. F. Ioffe, RAN. Retrieved 2014-01-01.
- ↑ L. I. Berger (1997). Semiconductor Materials. CRC Press. pp. 123–124. ISBN 0-8493-8912-7. Retrieved 2014-01-01.
- ↑ "Apple, Samsung Cellphone Filter Orders Lift Avago".
- ↑ "ACPF-7001: Agilent Technologies Announces FBAR Filter for U.S. PCS Band Mobile Phones and Data Cards". wirelessZONE. EN-Genius Network Ltd. 2002-05-27. Retrieved 2008-10-18.
- ↑ "A Gestural Interface for Smart Watches".
- ↑ Przybyla, R.; al, et (2014). "3D Ultrasonic Gesture Recognition". International Solid State Circuits Conference. San Francisco. pp. 210–211.
- ↑ Ahmadi, A; Hadipour, NL; Kamfiroozi, M; Bagheri, Z (2012). "Theoretical study of aluminium nitride nanotubes for chemical sensing of formaldehyde". Sensors and Actuators B: Chemical. 161 (1): 1025–1029. doi:10.1016/j.snb.2011.12.001.
- ↑ Ahmadi Peyghan, A; Omidvar, A; Hadipour, NL; Bagheri, Z; Kamfiroozi, M (2012). "Can aluminum nitride nanotubes detect the toxic NH3 molecules?". Physica E. 44: 1357–1360. doi:10.1016/j.physe.2012.02.018.
- ↑ Y. Taniyasu; et al. (2006). "An Aluminium Nitride Light-Emitting Diode with a Wavelength of 210 Nanometres". Nature. 441 (7091): 325–328. doi:10.1038/nature04760. PMID 16710416.
- ↑ http://www.sand9.com
- ↑ http://www.vespermems.com
External links
- Jaime Andrés Pérez Taborda; J.C. Caicedo; M. Grisales; W. Saldarriaga; H. Riascos. "Deposition pressure effect on chemical, morphological and optical properties of binary Al-nitrides". Optics. 69: 92–103. doi:10.1016/j.optlastec.2014.12.009.
| Salts and covalent derivatives of the Nitride ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NH3 | He(N2)11 | ||||||||||||||||||
| Li3N | Be3N2 | BN | β-C3N4 g-C3N4 |
N2 | NxOy | NF3 | Ne | ||||||||||||
| Na3N | Mg3N2 | AlN | SiN | PN P3N5 |
SxNy SN S4N4 |
NCl3 | Ar | ||||||||||||
| K3N | Ca3N2 | ScN | TiN | VN | CrN Cr2N |
MnxNy | FexNy | CoN | Ni3N | CuN | Zn3N2 | GaN | Ge3N4 | As | Se | NBr3 | Kr | ||
| Rb3N | Sr3N2 | YN | ZrN | NbN | β-Mo2N | Tc | Ru | Rh | PdN | Ag3N | CdN | In | Sn | Sb | Te | I | Xe | ||
| Cs3N | Ba3N2 | Hf3N4 | TaN | WN | Re | Os | Ir | Pt | Au | Hg3N2 | TlN | Pb | BiN | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | CeN | Pr | Nd | Pm | Sm | Eu | GdN | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac | Th | Pa | UN | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
