Acid dye
An acid dye is a dye which is a salt of a sulfuric, carboxylic or phenolic organic acid. The salts are often sodium or ammonium salts. Acid dyes are typically soluble in water and possesses affinity for amphoteric fibers while lacking direct dyes' affinity for cellulose fibers. When dyeing, ionic bonding with fiber cationic sites accounts for fixation of colored anions in the dyed material. Acids are added to dyeing baths to increase the number of protonated amino-groups in fibers.
Some acid dyes are used as food colorants.[1]
Uses
Fibers
In the laboratory, home, or art studio, the acid used in the dye-bath is often vinegar (acetic acid)[2] or citric acid.[3] The uptake rate of the dye is controlled with the use of sodium chloride. In textiles, acid dyes are effective on protein fibers, i.e. animal hair fibers like wool, alpaca and mohair. They are also effective on silk.[4] They are effective in dyeing the synthetic fiber nylon, but of minimum interest in dyeing any other synthetic fibers.
Medical
In staining during microscopic examination for diagnosis or research, acid dyes are used to color basic tissue proteins. In contrast, basic dyes are used to stain cell nuclei and some other acidic components of tissues.[5]
Description
Acid dyes are generally divided into three classes which depend on fastness requirements, level dyeing properties and economy. The classes overlap and generally depend on type of fiber to be colored as well as the process used.
Acid dyes affix to fibers by hydrogen bonding, Van der Waals forces[6] and ionic bonding. They are normally sold as the Sodium salt, therefore they are in solution anionic. Animal protein fibers and synthetic nylon fibers contain many cationic sites. Therefore, there is an attraction of anionic dye molecule to a cationic site on the fiber. The strength (fastness) of this bond is related to the tendency of the dye to remain dissolved in water over fixation to the fiber.
Structures
-1%2C2-diphenyldiazene_200.svg.png)
The chemistry of acid dyes is quite complex. Dyes are normally very large aromatic molecules consisting of many linked rings. Acid dyes usually have a sulfo or carboxy group on the molecule making them soluble in water. Water is the medium in which dyeing takes place. Most acid dyes are related in basic structure to the following:
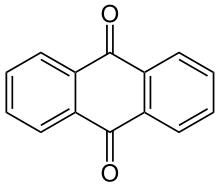
- Anthraquinone type: Many acid dyes are synthesized from chemical intermediates which form anthraquinone-like structures as their final state. Many blue dyes have this structure as their basic shape. The structure predominates in the leveling class of acid dye.
- Azo dyes: The structure of azo dyes is based on azobenzene, Ph-N=N−Ph (see image on right showing cis/trans isomers) Although azo dyes are a separate class of dyestuff mainly used In the dyeing of cotton (cellulose) fibers, many acid dyes have a similar structure, and most are red in color.
- Triphenylmethane related: Acid dyes having structures related to triphenylmethane predominate in the milling class of dye. There are many yellow and green dyes commercially applied to fibers that are related to triphenylmethane.
Classes of acid dyes
- Equalizing/leveling acid dyes: Highest level dyeing properties. Quite combinable in trichromatic shades. Relatively small molecule therefore high migration before fixation. Low wet fastness therefore normally not suited for apparel fabric.
- Milling acid dyes: Medium to high wet fastness. Some milling dyes have poor light fastness in pale shades. Generally not combinable. Used as self shades only.
- Metal complex acid dyes: More recent chemistry combined transition metals with dye precursors to produce metal complex acid dyes with the highest light fastness and wet fastness. These dyes are also very economical. They produce, however, duller shades.
Health and safety
Any dyes including acid dyes have the ability to induce sensitization in humans due to their complex molecular structure and the way in which they are metabolized in the body. This is extremely rare nowadays as we have a much greater understanding through experience and knowledge of dyestuffs themselves. Some acid dyes are used to color food. We wear fabrics every day exposing our skin to dyes.
The greatest risk of disease or injury due to dyes is by ingestion or exposure to dye dust. These scenarios are normally confined to textile workers. Whereby the dye itself is normally nontoxic, the molecules are metabolized (usually in the liver) where they may be broken back down to the original intermediates used in manufacture. Thus many intermediate chemicals used in dye manufacture have been identified as toxic and their use restricted. There is a growing trend among governments to ban the importation of dyes synthesized from restricted intermediates. For example: the dye CI Acid red 128 is banned in Europe as it was found to metabolize in the body back to ortho-toluidine, one of its chemical intermediates. Many intermediates used in dye manufacture such as ortho-toluidine, benzidine etc. were found to be carcinogenic. All the major chemical companies have now ceased to market these dyes. Some, however, are still produced but they are found to be totally safe when on the fiber in its final state. The use of these dyes is declining rapidly as cheap and safer alternatives are now easily available.
The incident concerning the dye Sudan 1 is an example of a suspected toxic dye finding its way into the food chain.
References
- ↑ Trowbridge Filippone, Peggy. "Food Color Additives". Retrieved September 8, 2016.
- ↑ "Acetic Acid". merriam-webster.com.
- ↑ "Citric Acid". merriam-webster.com.
- ↑ "How Acid Dye Works". Retrieved September 8, 2016.
- ↑ Bruckner, Monica Z. "Basic Cellular Staining". Retrieved December 12, 2013.
- ↑ Clark, Jim (2012). "Intermolecular bonding - van der Waals forces". chemguide.co.uk. Retrieved 15 June 2014.
