ATP synthase delta subunit
| ATP synthase delta (OSCP) subunit | |||||||||
|---|---|---|---|---|---|---|---|---|---|
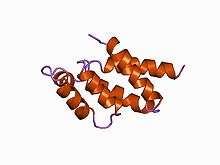 Structure of the N-terminal domain of the delta subunit of the E. coli ATPsynthase.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | OSCP | ||||||||
| Pfam | PF00213 | ||||||||
| InterPro | IPR000711 | ||||||||
| PROSITE | PDOC00327 | ||||||||
| SCOP | 1abv | ||||||||
| SUPERFAMILY | 1abv | ||||||||
| TCDB | 3.A.2 | ||||||||
| |||||||||
ATP synthase delta subunit is a subunit of bacterial and chloroplast ATPase, or OSCP (oligomycin sensitivity conferral protein) in mitochondrial ATPase (note that in mitochondria there is a different delta subunit, InterPro: IPR001469).
The OSCP/delta subunit appears to be part of the peripheral stalk that holds the F1 complex alpha3beta3 catalytic core stationary against the torque of the rotating central stalk, and links subunit A of the F0 complex with the F1 complex. In mitochondria, the peripheral stalk consists of OSCP, as well as F0 components F6, B and D. In bacteria and chloroplasts the peripheral stalks have different subunit compositions: delta and two copies of F0 component B (bacteria), or delta and F0 components B and B (chloroplasts).[2]
Human delta subunit of ATP synthase is coded by gene ATP5O.
References
- ↑ Wilkens S, Dunn SD, Chandler J, Dahlquist FW, Capaldi RA (March 1997). "Solution structure of the N-terminal domain of the delta subunit of the E. coli ATPsynthase". Nat. Struct. Biol. 4 (3): 198–201. doi:10.1038/nsb0397-198. PMID 9164460.
- ↑ Walker JE, Runswick MJ, Neuhaus D, Montgomery MG, Carbajo RJ, Kellas FA (2005). "Structure of the F1-binding domain of the stator of bovine F1Fo-ATPaseand how it binds an alpha-subunit". J. Mol. Biol. 351 (4): 824–838. doi:10.1016/j.jmb.2005.06.012. PMID 16045926.
Further reading
Wilkens, S.; Rodgers, A.; Ogilvie, I.; Capaldi, R. A. (1997). "Structure and arrangement of the delta subunit in the E. Coli ATP synthase (ECF1F0)". Biophysical chemistry. 68 (1–3): 95–102. doi:10.1016/s0301-4622(97)00018-5. PMID 9468613.
This article incorporates text from the public domain Pfam and InterPro IPR000711