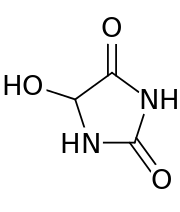
5-Hydroxyhydantoin
 | |
| Names | |
|---|---|
| IUPAC name
5-Hydroxy-2,4-imidazolidinedione | |
| Other names
Glyoxalurea; Allanturic acid | |
| Identifiers | |
| 29410-13-7 | |
| 3D model (Jmol) | Interactive image |
| |
| |
| Properties | |
| C3H4N2O3 | |
| Molar mass | 116.08 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5-Hydroxyhydantoin is an oxidation product of 2′-deoxycytidine. If not repaired, it may be processed by DNA polymerases that induce mutagenic processes.[1]
References
- ↑ "Excision of the oxidatively formed 5-hydroxyhydantoin and 5-hydroxy-5-methylhydantoin pyrimidine lesions by Escherichia coli and Saccharomyces cerevisiae DNA N-glycosylases". Biochimica et Biophysica Acta (BBA) - General Subjects. 1790: 16–24. doi:10.1016/j.bbagen.2008.10.001. Retrieved August 5, 2015.
This article is issued from Wikipedia - version of the 5/26/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.