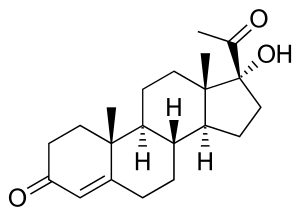
17α-Hydroxyprogesterone
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(8R,9S,10R,13S,14S,17R)-17-acetyl-17-hydroxy-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one | |
| Other names
17α-Hydroxypregn-4-ene-3,20-dione | |
| Identifiers | |
| 68-96-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:17252 |
| ChEMBL | ChEMBL1062 |
| ChemSpider | 6002 |
| ECHA InfoCard | 100.000.636 |
| 5104 | |
| PubChem | 6238 |
| UNII | 21807M87J2 |
| |
| |
| Properties | |
| C21H30O3 | |
| Molar mass | 330.46 g/mol |
| Melting point | 219.5 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
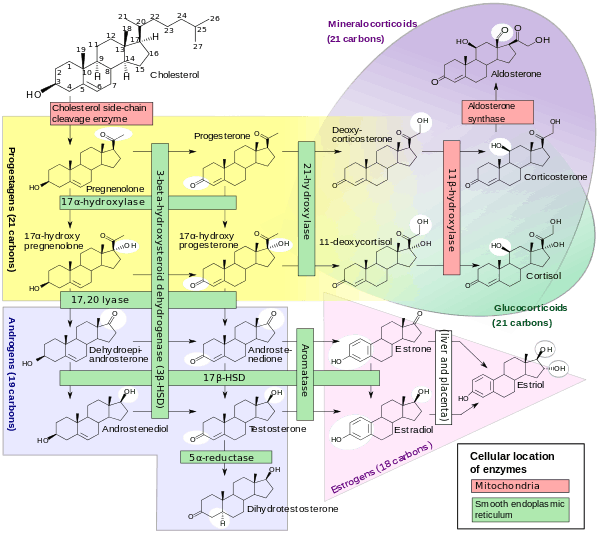
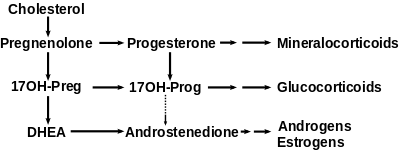
17α-Hydroxyprogesterone (17α-OHP), or hydroxyprogesterone (OHP) (INN, BAN), also known as 17α-hydroxypregn-4-ene-3,20-dione, is an endogenous progestogen steroid hormone related to progesterone.[1][2][3] It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.
Under the brand name Gestageno, 17α-OHP has been marketed for clinical use in Argentina.[3] However, esters of 17α-OHP, including hydroxyprogesterone caproate, as well as hydroxyprogesterone acetate and hydroxyprogesterone heptanoate to a much lesser extent, have been used far more widely in comparison, and when "hydroxyprogesterone" is referenced from the standpoint of medical use, what is usually being referred to is actually, in general, hydroxyprogesterone caproate.[1][2][3]
17α-OHP is also the parent compound of a class of progestins referred to as the 17α-hydroxyprogesterone derivatives.[4][5][6] Among others, this class of drugs includes chlormadinone acetate, cyproterone acetate, hydroxyprogesterone caproate, medroxyprogesterone acetate, and megestrol acetate.[4][5][6]
Production
17α-OHP is derived from progesterone via 17α-hydroxylase (encoded by CYP17A1), or from 17α-hydroxypregnenolone via 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase.
17α-OHP increases in the third trimester of pregnancy primarily due to fetal adrenal production.
This steroid is primarily produced in the adrenal glands and to some degree in the gonads, specifically the corpus luteum of the ovary. Normal levels are 3-90 ng/dl in children, and in women, 20-100 ng/dl prior to ovulation, and 100-500 ng/dl during the luteal phase.[7][8]
 |  |
| Steroidogenesis 1 | Steroidogenesis 2 |
Biological activity
17α-OHP is an agonist of the progesterone receptor (PR) similarly to progesterone, albeit weakly in comparison.[9] In addition, it is an antagonist of the mineralocorticoid receptor (MR) as well as a partial agonist of the glucocorticoid receptor (GR), albeit with very low potency (EC50 >100-fold less relative to cortisol) at the latter site, also similarly to progesterone.[9][10][11]
Measurement
Measurements of levels of 17α-OHP are useful in the evaluation of patients with suspected congenital adrenal hyperplasia as the typical enzymes that are defective, namely 21-hydroxylase and 11β-hydroxylase, lead to a build-up of 17α-OHP. In contrast, the rare patient with 17α-hydroxylase deficiency will have very low or undetectable levels of 17α-OHP. 17α-OHP levels can also be used to measure contribution of progestational activity of the corpus luteum during pregnancy as progesterone but not 17α-OHP is also contributed by the placenta.
Earlier immunoassays like RIA (radioimmunoassay) or IRMA (immunoradiometric assay) were used to clinically determine 17α-OHP. Today more sophisticated methods use gas or liquid chromatography and mass spectrometry (e.g. LC-MS/MS).
See also
- 11α-Hydroxyprogesterone
- 5α-Dihydroprogesterone
- 20-Dihydroprogesterone
- 11-Deoxycorticosterone
- 11-Deoxycortisol
- 17α-Methylprogesterone
- 19-Norprogesterone
- 19-Nortestosterone
References
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 664–665. ISBN 978-1-4757-2085-3.
- 1 2 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 146–. ISBN 978-94-011-4439-1.
- 1 2 3 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 532–. ISBN 978-3-88763-075-1.
- 1 2 Jeffrey K. Aronson (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 289–. ISBN 978-0-08-093292-7.
- 1 2 Robert Alan Prentky; Ann Wolbert Burgess (31 July 2000). Forensic Management of Sexual Offenders. Springer Science & Business Media. pp. 219–. ISBN 978-0-306-46278-8.
- 1 2 H. J. Smith; Hywel Williams (1 January 1983). Introduction to the Principles of Drug Design. Elsevier. pp. 187–. ISBN 978-1-4831-8350-3.
- ↑ Reference Values During Pregnancy
- ↑ normal ranges for hormone tests in women
- 1 2 Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN (2007). "Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins". Am. J. Obstet. Gynecol. 197 (6): 599.e1–7. doi:10.1016/j.ajog.2007.05.024. PMC 2278032
 . PMID 18060946.
. PMID 18060946. - ↑ Pijnenburg-Kleizen KJ, Engels M, Mooij CF, Griffin A, Krone N, Span PN, et al. (2015). "Adrenal Steroid Metabolites Accumulating in Congenital Adrenal Hyperplasia lead to Transactivation of the Glucocorticoid Receptor". Endocrinology: en20151087. doi:10.1210/en.2015-1087. PMID 26207344.
- ↑ Sun, Kang; Lei, Kaiyu; Chen, Li; Georgiou, Ektoras X.; Sooranna, Suren R.; Khanjani, Shirin; Brosens, Jan J.; Bennett, Phillip R.; Johnson, Mark R. (2012). "Progesterone Acts via the Nuclear Glucocorticoid Receptor to Suppress IL-1β-Induced COX-2 Expression in Human Term Myometrial Cells". PLoS ONE. 7 (11): e50167. doi:10.1371/journal.pone.0050167. ISSN 1932-6203.